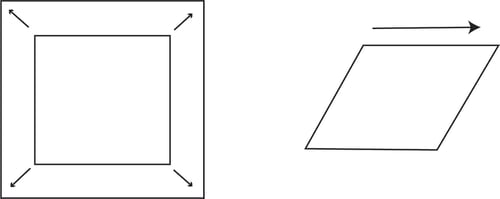
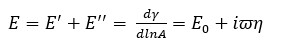The deformation of the interfacial layer can be caused either by changing the shape (shear) or the area (dilatational) of the interphase.
Shear interfacial rheology can be studied by using an interfacial shear rheometer. Dilatation methods on the other hand include the oscillating barrier method in Langmuir trough, or the pulsating drop method.
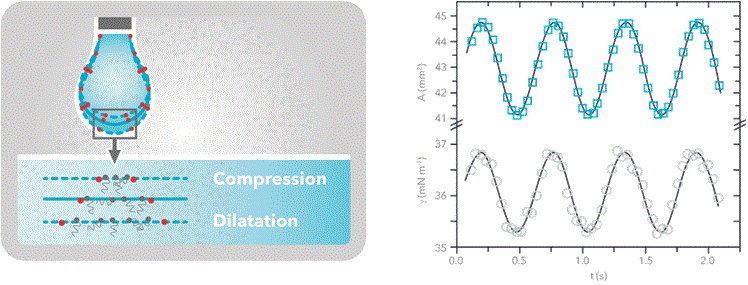
Pulsating drop method
Dilatational interfacial rheology can be studied with Theta pulsating drop. The measurement is based on the surface tension measurement by the pendant drop method. The pulsating module contains a piezo pump that is able to oscillate the volume of the droplet (and thus the surface area) with a known frequency. The interfacial tension will respond to that change since the distance between molecules at the interfacial layer will change with the same frequency.
From the measured interfacial tension data, the surface dilatation modulus E as well as its elastic E’ (storage) and viscous E’’ (loss) components can be calculated according to the equation
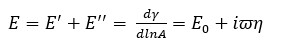
Oscillating barriers
Oscillating barriers is a dilatational interfacial rheology measurement done with the Langmuir trough with the help of moving barriers. Oscillating barrier measurement can be done with the same measurement hardware and software as your standard Langmuir isotherm.
Langmuir trough is a common method to study the properties of the monolayers. Most typically isotherms are measured which give information about the packing of the molecules at different surface pressures. Combining with the oscillating barrier measurement, one can define the viscoelastic properties of the interfacial layer at selected surface pressure. Oscillating barrier measurements are limited to low frequencies.
Interfacial shear rheometer
Interfacial rheology is a challenging field of research because the magnitude of forces in the interface is exceedingly small. Still, there are several different interfacial shear methods proposed which include the rotating ring, bicone, and magnetic needle methods. Especially the bicone method suffers from low sensitivity and thus limits the studies for macromolecular layers.
The KSV NIMA ISR Flip utilizes a small magnetic probe that is moved with an oscillating magnetic field. The method reduces the inertia and enhances the sensitivity of the probe compared to the rotating ring and bicone methods to enable the measurement of low molecular weight surface-active compounds.
Working principle
A magnetized probe is moved at an air-liquid or liquid-liquid interface using a magnetic field created by permanent magnets. The strength of the magnetic trap is controlled by moving the trap up and down. The probe movement is recorded with the help of a high-resolution camera which can be placed either below or above the interface. The viscoelastic properties of the surface can be calculated from the combination of the trap and the probe movement. Surface viscoelasticity can be divided into elastic and viscous properties of the thin film.
In addition to a so-called manual mode, three automated measurement modes are available:
- Frequency sweep allows the measurement of the viscoelastic properties at different frequencies. This method is extremely useful since rheology is time-dependent. It allows the definition of the rheological behavior of thin films in different time scales and provides information about the dominance of viscosity or elasticity.
- Single frequency measurement can be used to define the time dependency of viscoelastic properties. When combined with the ISR Flip high compression trough the viscoelastic changes can be defined as a function of surface pressure.
- Amplitude sweep measurement allows defining the linear region of the viscoelastic film and allows defining suitable oscillation amplitude for different thin films. Shear-thinning and thickening can also be detected with this method.
One of the additional benefits of the ISR Flip is that it can be readily connected with the ISR Flip high compression trough which enables measurements at controlled packing densities. Moving barriers will compress the monolayer at the interface while highly sensitive balance detects the surface pressure changes as a function of surface area. From the Langmuir isotherm different phases of the film can be seen, and viscoelastic properties determine as a function of layer packing density.