With increasing concern about global warming and its effects on our planet, the storage of greenhouse gases – mainly CO2 – has become a topic of many research groups. The safe trapping of carbon dioxide in deep saline aquifers is considered as a viable means of controlling the emission of carbon to the atmosphere.
One of the potential subsurface systems considered for geological sequestration involves deep saline aquifers. For successful CO2 sequestration, the long-term trapping of CO2 needs to be ensured. This requires an understanding of all physical and chemical trapping mechanisms by which CO2 may be retained. CO2 trapping below an impermeable confining layer, or the immobilization of CO2 in aquifer pore spaces, have the largest and most immediate impact. The effectiveness of both trapping mechanisms is dependent on capillary pressure, and thus interfacial tension properties between CO2 -brine and CO2-rock play an important role. Understanding these interactions requires measurements at high temperatures and pressures to mimic the reservoir conditions.
CO2-brine interfacial tension has been studied as a function of pressure, temperature, salt composition and concentration. Figure 1 presents typical interfacial tension measurement data showing a plateau of the interfacial tension value at pressure around 120 bar.
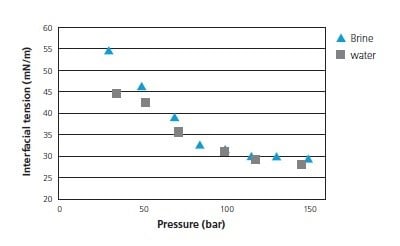
Figure 1: Interfacial tension between CO2-water and CO2-brine (35,000 ppm) as a function of pressure (at 45 °C)
Wettability is often defined as the tendency of one fluid to spread and adhere to a solid surface in the presence of another immiscible fluid. In the case of aquifers, a brine-CO2-rock system needs to be considered. If the rock is water-wet, there is a tendency for the brine to occupy the small pores and to contact most of the rock. Similarly, if the injected CO2 eventually wets the rock, it will occupy the small pores and contact most of the rock. Changes in wettability have been studied by measuring the contact angle of CO2 on mineral surfaces in brine at elevated pressures and temperatures.
