Absorption
Absorption is a chemical or physical process in which molecules, atoms or ions penetrate into bulk that can be solid, liquid or gas. Absorption should not be confused with adsorption.
Absorption of liquid into solid substrate or powder can be studied with optical or force tensiometers.
Adhesion
Adhesion is the attraction between two dissimilar phases. Current theory divides adhesion into the three categories: fundamental adhesion, practical adhesion and thermodynamic adhesion.
- Fundamental adhesion is the sum of all interfacial intermolecular interactions between the two phases. It corresponds to the energy required to break chemical bonds at the weakest plane of the two-phase system. The fundamental adhesion cannot be directly measured since there are always other factors affecting in a measurement system.
- Practical adhesion is a function of fundamental adhesion and other factors, including internal stresses and the error caused by the measurement method. Practical adhesion can be measured by using variety of different techniques, such as Scotch tape test or stylus method.
- Thermodynamic adhesion is defined as the reversible work done in creating a unit area of the interface between two phases (see figure below). Typically, the unit is mN/m.
WAB = γA + γB – γAB where WAB is the thermodynamic work of adhesion, γA the surface tension of substance A, γB the surface tension of substance B and γAB the interfacial free energy.
For two solid phases, the work of adhesion is not useful since there are various unknown parameters in the equation. If, however, one of the phases is liquid and the other one solid, the work of adhesion can be defined with Young-Dupré equation:WAB = γB (1 + cos θ) where γB is the surface tension of the liquid and θ is the contact angle between the liquid and solid. Now, the work of adhesion can be calculated by measuring surface tension of the liquid and contact angle with Attension tensiometers.
Adsorption
Adsorption is adhesion of molecules, atoms or ions on the surface. Adsorption should not be confused with absorption.
Controlling the adsorption or rejection of certain types of materials at solid surfaces or liquid interfaces is very important for numerous applications, such as biocompatible surfaces, sensor technologies, cleaning processes as well as emulsion and foam stability.
Adsorption of materials to solid surfaces is highly dependent on the interaction between the solid material as well as the adsorbate, while surface-active agent properties largely influence the behavior and properties of liquid interfaces.
The speed of adsorption of surface-active agents to liquid or solid interfaces plays a key role in formulations for industrial cleaning processes, as well as stabilization of emulsions and foams for paint, pharmaceutical, cosmetic, food and mineral processing applications.
Advancing contact angle
Advancing contact angle is the contact angle that is measured when the three-phase point is advancing. More information about measurement of advancing contact angle can be found here.
Brewster angle microscopy
Brewster angle microscopy is used for visualization of floating monolayers at air-water interface.
The working principle of Brewster angle microscopy is very simple. A p-polarized light beam is directed to the air-water interface at the Brewster angle. For the air-water interface, the angle is 53 ° which can be easily calculated from the reflective indexes of the phases.
Tan α = n2/n1
For air n1 = 1 and water n2 ≈ 1.33. When the light hits the areas where no thin film is present, there is no reflection and the image is seen as black. When even just a monolayer is on the interface, the Brewster law is not fulfilled, and the reflection occurs.
Captive bubble
Captive bubble is a contact angle measurement method where the solid being measured is immersed into liquid (usually water) and air bubble or oil drop is used as a drop phase. Captive bubble method is especially used for contact angle measurements on contact lenses and for contact angle measurements in oil related applications.
Cassie-Baxter equation
Cassie - Baxter states that the apparent (i.e. measured) contant angle on a porous surface is influenced by the area fraction of the solid vs. the area of the pores.
cosθA= -1 + fS (cosθS+ 1)
Equation is valid when the pores of the material are not wetted i.e. the liquid doesn't penetrate into the pores. This is a basis of the extreme superhydrophobic properties of the materials.
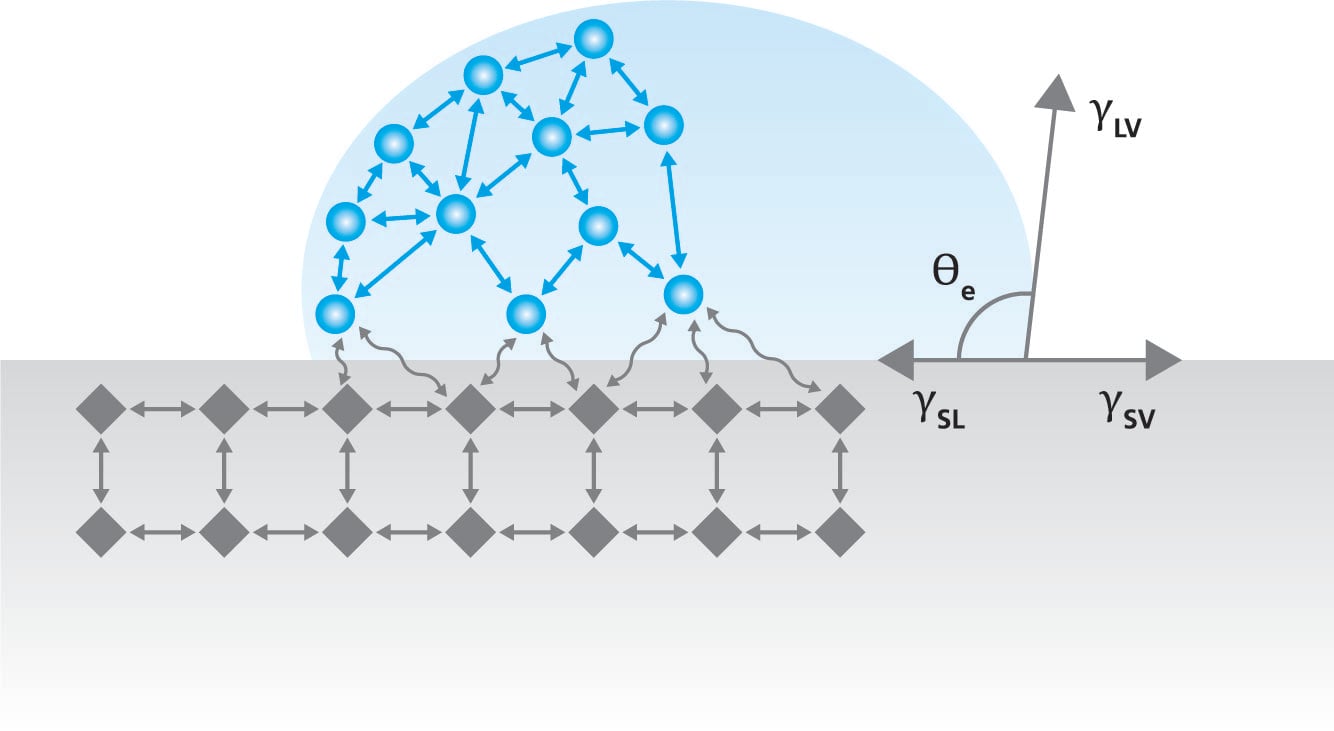
Contact angle
Contact angle, θ, is a quantitative measure of wetting of a solid by a liquid. It is defined geometrically as the angle formed by a liquid at the three-phase boundary where a liquid, gas and solid intersect. The well-known Young equation describes the balance at the three-phase contact of solid-liquid and gas.
γsv = γsl + γlv cos θY
The interfacial tensions, γsv, γsl and γlv, form the equilibrium contact angle of wetting, many times referred to as the Young contact angle, θY. Contact angles can be divided into static and dynamic and roughness corrected contact angles.
Contact angle goniometer
Contact angle goniometer is also called optical tensiometer and is used to measure contact angle, surface and interfacial tension and surface free energy.
Contact angle hysteresis
Contact angle hysteresis is the difference between the maximum (advancing) and minimum (receding) contact angles. The significance of hysteresis has been the object of much research and can be used to help characterize chemical heterogeneity, roughness and mobility.
Contact angle hysteresis arises from the chemical and topographical heterogeneity of the surface, solution impurities absorbing on the surface, or swelling, rearrangement or alteration of the surface by the solvent. Advancing and receding contact angles give the maximum and minimum values that the static contact angle can have on the surface.
Contact angle meter
Contact angle meter (also called optical tensiometer) is used for measurement of surface tension, interfacial tension, contact angle and surface free energy.
Critical micelle concentration
Critical micelle concentration (CMC) is defined as the concentration above which micelles form. At low surfactant concentration, the surfactant molecules arrange on the surface. When more surfactant is added the surface tension of the solution starts to rapidly decrease since more and more surfactant molecules will be on the surface. When the surface becomes saturated, the addition of the surfactant molecules will lead to formation of micelles. This concentration point is called critical micelle concentration.
Dip coating
Dip coating is the precision controlled immersion and withdrawal of any substrate into a reservoir of liquid for the purpose of depositing a layer of material. Many chemical and nanomaterial engineering research projects in academia and industry make use of the dip coating technique.
Drop shape analyzer
Drop shape analyzer is an other name for optical tensiometer used to measure contact angle, surface and interfacial tension and surface free energy.
Du Noüy ring method
Du Noüy ring method is used the measure surface tension of liquids and interfacial tension between two liquids. The method is based on the very sensitive microbalance to which a probe i.e the du Noüy ring is hang. The ring is then submerged in the liquid and pulled back up, which will cause a meniscus to be pulled with the ring. The surface tension (or interfacial tension) is determined based on the mass of liquid pulled with the ring.
Dynamic contact angle
When the three-phase boundary is moving, dynamic contact angles can be measured, and are referred as advancing and receding angles. Contact angle hysteresis is the difference between the advancing and receding contact angles.
Dynamic contact angles and contact angle hysteresis has become a popular topic because of the recent interest in novel super-hydrophobic and self-cleaning surfaces. This is important since small roll-off angles are needed for self-cleaning applications. Hysteresis is however also important in other applications such as intrusion of water into porous media, coating, and adsorption at liquid/solid interface.
There are three methods to measure the advancing and receding contact angles, i.e. dynamic contact angles, and thus contact angle hysteresis; tilting method, needle method and Wilhelmy plate.
Force tensiometry
Force tensiometry is a powerful and accurate technique to measure static surface tension and interfacial tension of liquids. These direct measurements allow determination of material and surface properties, such as dynamic contact angle, surface free energy.
Force tensiometry provides information necessary for the control, development and modification of liquid and solid surfaces. It enables precise characterization of a number of material properties. Analysis of surface/interfacial tension and contact angles provides valuable information on the interactions between gas, liquid and solid phases. These interactions play a key role in the study of:
- Wettability
- Sorption
- Formulation
- Surfactant development
- Adhesion
Force tensiometry is the method of choice for many industrial standards related to characterization of liquids. It is used in the testing and quality control of insulator and transformer oils in compliance with the standard, ASTM-D971. Force tensiometry is also the most used technique for measuring critical micelle concentration (CMC) for the optimization of surfactant concentration. In addition, it is the only method available for determining the absorption and contact angle of packed powder, pigments or fiber beds with the Washburn method. It is also commonly used for single fiber measurements.
The basic principle of all force tensiometry experiments is to record and analyze the forces exerted onto a probe or solid sample using a sensitive microbalance.
Goniometer
Goniometer is an instrument that measure an angle or allows an object to be positioned in an angle. In surface science contact angle measurements are done with instruments that are sometimes called contact angle goniometers.
Interfacial tension
Interfacial tension is the property between any two substances. Whereas surface tension is the property of the liquid and measured between gas-liquid (typically air-liquid) interface, the interfacial tension is similar but between liquid-liquid (immisible liquids) or liquid-solid interface.
Interfacial rheology
Interfacial rheology studies the rheological properties of the interfaces. When surface active molecules are present, they tend to adsorp at air-liquid or liquid-liquid interfaces forming an interfacial layer. Interfacial rheology studies the properties of this interfacial layer such as interfacial elasticity and viscosity. These are important in the many industrial product such as emulsions.
Langmuir Film
A Langmuir film can be defined as an insoluble spread monolayer of atoms or molecules floating at the liquid-gas interface (or liquid-liquid). Monolayer formation is possible due to the forces of self-assembly on insoluble molecules at the surface of a liquid.
Langmuir-Blodgett Film
Langmuir-Blodgett technology enables the deposition of single- or multimolecular layers from a liquid surface onto a solid substrate with excellent film structure control. LB is especially well suited for creating highly organized nanoparticle coatings, for example.
Langmuir-Blodgett film (or LB film) can be defined as one or more monolayers of material deposited from a liquid surface onto a solid substrate by dipping the substrate through a floating monolayer at a constant molecular density. LB films are formed by one or several Langmuir films deposited onto a solid surface by vertical dipping of the solid substrate from the gas phase into the liquid phase (or vice versa).
The films obtained by this process can be highly organized, ranging from ultrathin monolayer to multilayer structures built up of hundreds of monolayers.
Langmuir-Schaefer Film
Langmuir-Schaefer film (or LS film) can be defined as one or more monolayers of material deposited from a liquid surface onto a solid substrate. The deposition can be made by dipping the substrate horizontally through a floating monolayer at a constant molecular density or by placing the substrate in contact with the monolayer. LS deposition can be used for example to create nanoparticle coatings with controlled packing density.
Langmuir-Schaefer films formed by one or several Langmuir films deposited onto a solid surface by horizontally dipping (rather than vertically) the solid substrate from the gas phase to the liquid phase or from the liquid phase through the gas phase. The films obtained can be highly organized ranging from ultrathin monolayer to multilayer structures built up of hundreds of monolayers.
The Langmuir-Schaefer technique is derived from the Langmuir-Blodgett technique, Langmuir-Schaefer films being very similar to Langmuir-Blodgett films.
Both methods can be used in creating highly organized nanoparticle coatings with controlled packing density and film thickness.
Microscopy trough
A microscopy trough is a special kind of Langmuir trough for characterization of Langmuir films before, during and after fabrication.
A microscopy trough is a special kind of Langmuir trough that includes a sapphire window in the top base of the PTFE trough that allows high optical transmission down to a wavelength of 200 nm (suitable for visible light or UV microscopy). It enables characterization of Langmuir films before, during and after fabrication. Troughs suitable for both upright and inverted microscopes are available.
UV-visible absorption spectroscopy of monolayers on the Langmuir Trough can be used to determine how light-absorbing molecules interact with one another when a monolayer of the molecules, or mixtures of molecules, are compressed to various surface pressures. The molecular neighbor-neighbor separation and packing density is made possible by controlling the surface pressure of the monolayer.
When photoluminescent materials are studied, the Inverted Microscopy Trough allows a microscope objective lens to reach within 2.2 mm of the monolayer, from beneath, to enable stimulation of the monolayer with very low wavelength light from above. The transmission properties of the sapphire microscopy window and the glass inverted microscopy window are shown below.
Monolayer
A monolayer is a single-molecule thick film or layer of closely packed atoms or molecules at an interface.
Monolayers are often formed at the air-water interface as a Langmuir film. Monolayers are often located on a solid surface or floating on a gas-liquid interface. However, some monolayers can also be formed at a liquid-liquid interface.
Many materials can be used to form monolayers including lipids, nanoparticles, polymers and proteins to name a few. Monolayers are expected to be highly useful components in many practical and commercial applications such as sensors, detectors, displays and electronic circuit components.
Optical goniometer
Optical goniometer is an other name for optical tensiometer.
Optical tensiometer
Optical tensiometry is a versatile technique used to characterize material surface properties and interfacial interactions between gas, liquid and solid phases. Optical tensiometers are used in R&D and quality control in a variety of industries, including biomaterials, chemicals, pharmaceuticals, electronics, foods, energy, environment, paper and packaging.
Optical tensiometers are primarily developed for the measurement of contact angles and surface free energy. They are also capable of determining surface tension, interfacial tension, 3D surface roughness and interfacial rheology.
Optical tensiometers capture drop images and automatically analyze the drop shape as a function of time. The drop shape is function of surface tension of liquid, gravity and the density difference between sample liquid and the surrounding medium. On a solid, the liquid forms a drop with a contact angle that also depends on the solid’s surface free energy. The captured image is analyzed with a drop profile fitting method to determine contact angle and surface tension.
Receding contact angle
Receding contact angle is the contact angle that is measured when the three-phase point is receding.
Roll-off angle
The roll-off angle is the inclination angle of the surface at which the droplet rolls off the surface.
Roll-to-roll Langmuir-Blodgett
Roll-to-roll Langmuir-Blodgett is a Langmuir-Blodgett deposition technique where a thin polymer film is continuously coated. It enable high-throughtput deposition on a flexible substrate.
Roughness corrected contact angle
The relationship between roughness and wettability was defined in 1936 by Wenzel, who stated that adding surface roughness would enhance the wettability caused by the chemistry of the surface. For example, if the surface is chemically hydrophobic, it will become even more hydrophobic when surface roughness is added. Wenzel statement can be described with equation below.
cos θm = r · cos θY
Where θm is the measured contact angle, θY is the Young contact angle and r is the roughness ratio. The roughness ratio is defined as the ratio between the actual and projected solid surface area (r = 1 for a smooth surface and r > 1 for a rough one) and can be calculated from a 3D roughness parameter Sdr as shown already. It is important to notice that the Wenzel equation is based on the assumption that the liquid penetrates into the roughness grooves. It has been stated that if the droplet is larger than the roughness scale by two to three orders of magnitude, the Wenzel equation applies. Wenzel corrected contact angles have been utilized for example to study the wettability of paper sheets and cell adhesion to biomaterial surfaces. Both micro and nanoscale roughness have been shown to have influence on surface wettability.
Spreading
Dynamic wetting of liquid on a solid surface can be divided into a spreading and absorption that can both be followed by contact angle measurements. Knowing the spreading rate is essential in many application segments, for example in the textile industry, coating and ink development and in detergent formulation.
The shape of a liquid front in contact with a solid substrate is determined by the interfacial forces of the participating phases (gas/liquid, gas/solid, liquid/solid, liquid/liquid). Wettability of a surface by a liquid is the actual process of spreading. Wetting can qualitatively determined with by measuring contact angles, i.e. with low contact angles indicating good wetting, and high contact angles indicating non-wetting conditions. A quantitative measure of wetting is the spreading coefficient, which helps in predicting whether or not a liquid spreads spontaneously on a solid or another liquid. The spreading coefficient is the energy difference between the solid substrate with the contacting gas and liquid phases.
Static contact angle
Static contact angles are measured when a droplet is standing on the surface and the three-phase boundary is not moving. This is still the most measured contact angle value.
Spin coating
Spin coating is a technique where rotation is used to spread material on the solid substrate. The material is typically pipetted on the center of the substrate after which different acceleration and spin speed steps are used to spread the material. Spin coating is widely used in semiconductor industry to spread the photoresist on the silicon wafer.
Surface free energy
Surface free energy of a solid is equivalent to surface tension of a liquid and the unit is the same mN/m (dynes/cm).
Surface Heterogeneity
Typically, coatings and surface treatments should be homogeneously distributed over the whole surface that they applied to, and at times surface heterogeneity can be a desirable characteristic.
After applying any coating or surface treatment, heterogeneity control can be performed by contact angle measurement. Contact angle is one of the most sensitive of all surface analytical techniques since even the top nanometer of the surface influences wetting behavior. As a simple and fast measuring technique, contact angle is commonly utilized to study surface heterogeneity.Depending on the application, the contact angle measurement can be applied to study surface heterogeneity in different length scales:
- Macroscale heterogeneity and cleanliness can be studied by contact angle mapping.
- Contact angle hysteresis can be utilized to define nanoscale surface heterogeneity. Surface heterogeneity is particularly important for the coating of super-hydrophobic surfaces where nanoscale roughness is one of the major reasons for the so-called lotus effect.
Surface Pressure
In the context of a Langmuir film, surface pressure is the difference in surface tension measured between a clean sub-phase and a monolayer-covered sub-phase. Surface pressure greatly influences monolayer properties.
The surface pressure can be presented as
Π=ϒ-ϒ0
where ϒ0 is the surface tension of the sub-phase in absence of a monolayer and ϒ is the surface tension with the monolayer present at the interface.
Surface pressure - area isotherm
Surface pressure—area isotherm or π-A isotherm can be defined as a measurement at constant temperature of surface pressure, as a function of the available area for each molecule in a floating monolayer (Langmuir film).
The most important indicator of the monolayer properties of an amphiphilic material is found by measuring the surface pressure as a function of the area of water surface available to each molecule. Usually an isotherm is recorded at constant temperature by compressing the film (with the barriers) at a constant rate while continuously monitoring the surface pressure. Depending on the material, repeated compression and expansion may be necessary to achieve a reproducible trace.
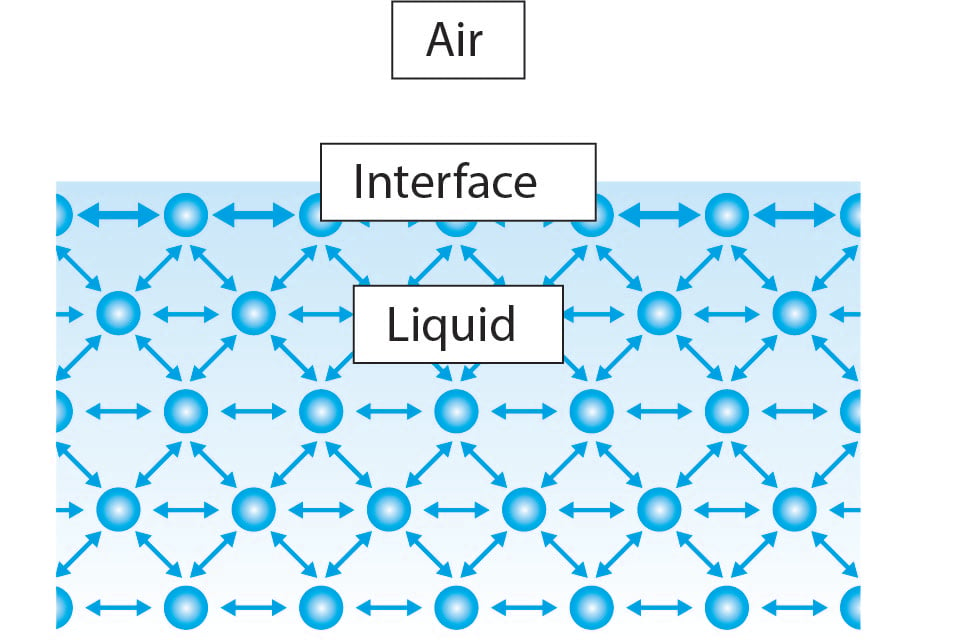
Surface tension
Surface tension is a measurement of the cohesive energy present at an interface. The molecules of a liquid attract each other. The interactions of a molecule in the bulk of a liquid are balanced by an equal attractive force in all directions. The molecules at the surface of a liquid experience an imbalance of forces as shown below.
The air/water interface (and other gas-liquid interfaces) possesses excess free energy originating from the difference in environment between the surface molecules and those in the bulk. This interfacial free energy is responsible for the surface tension.
Surfactant
Surfactants are compounds that lower the surface tension of the liquid, the interfacial tension between two liquids or interfacial tension between a liquid and solid. Surfactants can act as detergents, wetting agents, emulsifiers, foaming agents and dispersants. Surfactants are amphiphilic molecules that contain the hydrophobic hydrocarbon tail and hydrophilic head.
Washburn equation
Washburn equation can be written as
w2=(ctρ2γlcosθ) /2η
, where w is the accumulated mass during time t. r is the density of the liquid,γl is the surface tension of the liquid, η is a viscosity of the liquid, θ is the contact angle between the solid and the liquid and c is the material constant. Washburn equation is used to determine the powder wettability.
Wenzel equation
Wenzel equation states that the surface roughness will enhance the underlying wettability of the surface.
Wenzel equation can be written as
cosΘm = rcosΘY
where Θm is the measured contact angle, ΘY is Young’s contact angle and r is the roughness ratio. Roughness ratio is defined as the ratio between the actual and projected solid surface area (r=1 for a smooth surface and > 1 for a rough surface). It is important to note that the Wenzel equation is based on the assumption that the liquid completely penetrates the roughness grooves.
Wettability
Wettability or wetting is the process that occurs when a liquid spreads and/or absorbs on a solid substrate or material. Wettability can be estimated by measuring the contact angle or by calculating the spreading coefficient.
Examples of where solid surface wettability plays a crucial role include: body implants, contact lenses, biocompatibility, printing processes, packaging, semiconductor wafers, electronic products, biofilm growth, fabrics, super-hydrophobic surfaces, self-cleaning and non-stick surfaces. In addition, the wettability of smaller objects such as, fibers, micro- and nanoparticles play an important role in stabilization and performance of many products, such as: composites, paints and coatings, inks, cosmetics, pharmaceuticals, and food products. Wetting of a liquid on a solid surface depends on the solid surface properties as well as the liquid used. By manipulating the properties of surfaces the function or performance of a solid surface or material can be optimized for the purpose of interest. If modifying the solid surface properties is difficult, then try modifying the properties of the liquid to achieve the desired wetting characteristics. Contact angle is the primary parameter for determining wettability.
Wilhelmy plate method
Wilhelmy plate is a thin pate typically made of platinum. The wilhelmy plate method is used to measure surface (and interfacial tension) of liquid. The method is based on a very sensitive microbalance where the probe i.e. the Wilhelmy plate is hang. The plate is immersed into the liquid and pulled back up which will cause a meniscus of liquid be pulled with the plate. If the perimeter of the plate is known and the zero contact angle between the plate and the liquid is assumed, the surface tension of the liquid can be calculated based on the mass of liquid the plate is pulling up. The method can also be used to determine the contact angle of the regular objects if the surface tension of the liquid is known instead.
Work of adhesion
Work of adhesion is defined as a work required to separate two phases from each other. When the two phases are in contact with each other, there is an interface between them with a certain interfacial energy, γAB. When the two phases are separated, this interface will disappear but two new are formed. These are the interface between phase A and air (γA) and the interface between phase B and air (γB). The work that is required to separate these is work of adhesion and described by Dupré as: WAB = γA + γB – γAB.
Young equation
The well-known Young´s equation describes the balance at the three-phase contact where gas, liquid and solid meet.
Young equation can be written as
γsv = γsl+ γlvcosθY
The interfacial tensions, γsv, γsl, and γlv, form the equilibrium contact angle of wetting, many times referred to Young´s contact angle θY.

