Adhesion force characterizes the force that is required to detach a liquid droplet from a surface that it contacts. It provides a way to quantify the attractive interaction between liquid and solid.
The adhesion force of a surface to liquid depends on surface wettability of the surface, the volume of liquid droplet, liquid surface tension, and the measuring procedure employed. At given measuring conditions (i.e. fixed liquid volume and measuring procedure), the adhesion force is determined by contact angle of the surface; more specifically, by the receding contact angle of the surface. Receding contact angle is the critical contact angle below which the solid-liquid-gas contact line retraction is initiated. A surface with larger receding contact angle exhibits lower adhesion force.
The measuring method of adhesion force is schematically shown in below. A liquid droplet attached to a micro-balance is first made to contact with the sample surface, and then is separated slowly from it. During this process, the force-displacement curve is recorded by the micro-balance. The adhesion force is measured as the pull-off force when separating the surface and the droplet. The next figure shows a typical force-displacement curve obtained from a hydrophobic surface. The adhesion force between the surface and a water droplet is shown as the pull-off force

Experimental setup for measuring the adhesion force.
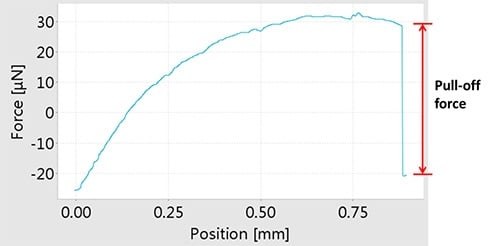 Force-displacement curve obtained from a hydrophobic surface and a water droplet. The red arrow indicates the pull‑off force.
Force-displacement curve obtained from a hydrophobic surface and a water droplet. The red arrow indicates the pull‑off force.
Adhesion force can be used to characterize liquid repellency of self-cleaning surfaces as surfaces with low solid-liquid adhesion force are more suitable for self-cleaning applications. It can also give information on the wetting states of micro-textured surfaces that exhibit large apparent contact angles. For example, a surface in Wenzel type wetting state (a) usually shows large adhesion force whereas a surface in Cassie-Baxter state (b) is expected to exhibit much lower values
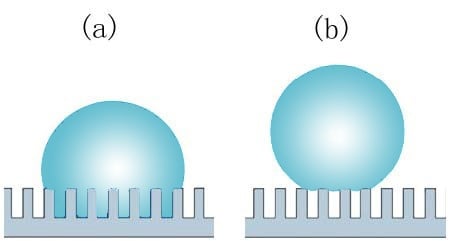
Two different wetting states. (a) Wenzel state, and (b) Cassie-Baxter state.
Our tensiometer selector guides you to your optimal instrument.
Try the instrument selector
