Fundamentals and Working Principles
EQCM-D measures two parameters via QCM-D, the resonance frequency (f) and energy dissipation (D) of the quartz crystal sensor, where the frequency relates to mass changes at the surface and the dissipation relates to the softness or viscoelasticity of the surface-adhering layer. By applying an electrical potential, electrochemical reactions can be induced, and the resulting mass and viscoelastic changes can be monitored in real-time.
The two methods, electrochemistry and QCM-D are ideally suited to be paired since both are surface-based techniques. QCM-D can provide real-time information on mass and structure of thin films in the form of changes in frequency or dissipation, while electrochemistry can be the stimulus of an interaction or provide information about interfacial charge transfer.
QSense EQCM-D is a so-called multi-harmonic EQCM-D, which measures f and D at multiple harmonics, and allows for analysis of interfacial processes with nano-level sensitivity.
Design of the Electrochemical Cell
The electrochemical cell used in EQCM-D experiments typically consists of three electrodes: the working electrode (WE), the reference electrode (RE), and the counter electrode (CE), Fig. 1. The quartz crystal sensor acts as the working electrode, where the electrochemical reactions and mass changes are monitored.
- Working Electrode (WE): The quartz crystal sensor serves as the working electrode. It is coated with a conductive material, such as gold, to facilitate electrochemical reactions.
- Reference Electrode (RE): The reference electrode is usually a standard electrode, such as Ag/AgCl, which provides a stable reference potential against which the working electrode's potential is measured.
- Counter Electrode (CE): The counter electrode is typically made of an inert material, such as platinum, and completes the electrical circuit by allowing current to flow between the working and counter electrodes.
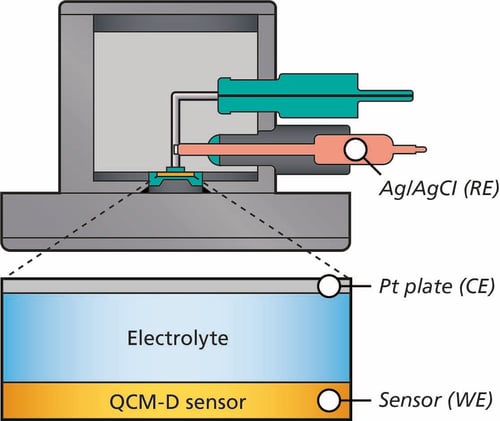
Figure 1: Schematic illustration of the cross section of QSense EQCM-D module showing the 3-electrode configuration.
Voltammetry
A typical EQCM-D setup combines cyclic voltammetry (CV) with QCM-D, enabling simultaneous measurements of frequency (f), dissipation (D), and current (I) in response to a varying potential (E) on the sensor surface.
In CV, the potential of the working electrode is cycled, and the resulting current is measured, allowing for the study of redox reactions and the corresponding mass changes at the electrode surface. This approach allows for a direct correlation between QCM-D and electrochemical results, Fig. 2. For example, in the study of battery electrode materials, CV can help identify the specific ions involved in the charge storage mechanism and their impact on the electrode's mass and viscoelastic properties.

Figure 2. EQCM-D measurement of copper being deposited onto and stripped from an Au sensor. Copper sulfate solution (10 mM CuSO4 in 0.1 M H2SO4) is injected into the QEM module whereafter five CV cycles are performed, starting at +0.3 V and cycling back and forth from -0.5 V to +0.5 V at 50 mV/s. The QCM-D data shows a reversible process where the decrease in f indicates mass building up on the surface while the increase in D indicates the film’s viscoelastic character.
Electrochemical Impedance Spectroscopy (EIS)
Another technique that can be combined with QCM-D is Electrochemical Impedance Spectroscopy (EIS). EIS measures the impedance of the electrochemical system over a range of frequencies, providing insights into the charge transfer processes and the properties of the interfacial layer. This technique is particularly useful for studying the properties of thin films and interfacial layers in various electrochemical systems.
In the study of lipid membranes, for example, the combination of QCM-D and EIS can provide a comprehensive understanding of the structural and functional properties of the membrane and reveal for example membrane disruption.




