
In the pursuit of safe, stable, and cheap energy storage, new battery materials are continuously being explored. In this quest, aqueous zinc ion batteries (AZIBs) are being evaluated as alternatives to alkaline rechargeable zinc batteries. In this post, we describe a user case where QSense EQCM-D, Electrochemical Quartz Crystal microbalance with Dissipation monitoring, was used to explore the reaction mechanisms of organic electrode materials with the ambition to support the development of AZIBs.
There are several aspects of AZIBs that make them an attractive alternative to alkaline rechargeable batteries. One benefit is that they work at near-neutral pH, which makes dendrite formation that can result in severe battery failure, less likely to occur compared to their alkaline counterpart. Also, as the cathode materials do not store protons, the pH of the electrolyte is maintained throughout the charge-discharge process.
Different AZIB cathode materials are currently being explored. Since the ion species stored at the cathode is important - the material needs to be able to store zinc ions - the charge storage mechanism is one aspect that must be assessed for the cathode material to be viable in practical battery applications.
QSense EQCM-D is a time-resolved surface sensitive technology which is used to characterize molecular interactions and reactions at surfaces and interfaces while monitoring electrical properties through electrochemical measurements. Such processes can be monitored with nano-level sensitivity via two parameters, frequency (Δf) and energy dissipation (ΔD) at multiple harmonics, so called multi-harmonic EQCM-D, where the frequency relates to mass changes at the surface and the dissipation relates to the softness or viscoelasticity of the surface-adhering layer.
Monitoring changes in mass and viscoelasticity, the method can provide quantitative information of ions stored in redox-active materials, such as battery electrode materials.
In this case study,1 the researchers wanted to identify which ions that are stored in an AZIB cathode material candidate, a quinone polymer, poly(benzoquinonyl sulfide) (PBQS).
Specifically, the goal was to answer the questions:
The In order to investigate the charge storage mechanism of the PBQS in a near-neutral zinc electrolyte solution and to address the questions asked, QSense EQCM-D analysis was combined with in situ FTIR, where this case study focuses on the former method (for additional details, see ref. 1). A schematic illustration of the EQCM-D setup and system design is shown in Fig. 1. The two-electrode configuration used was according to the below
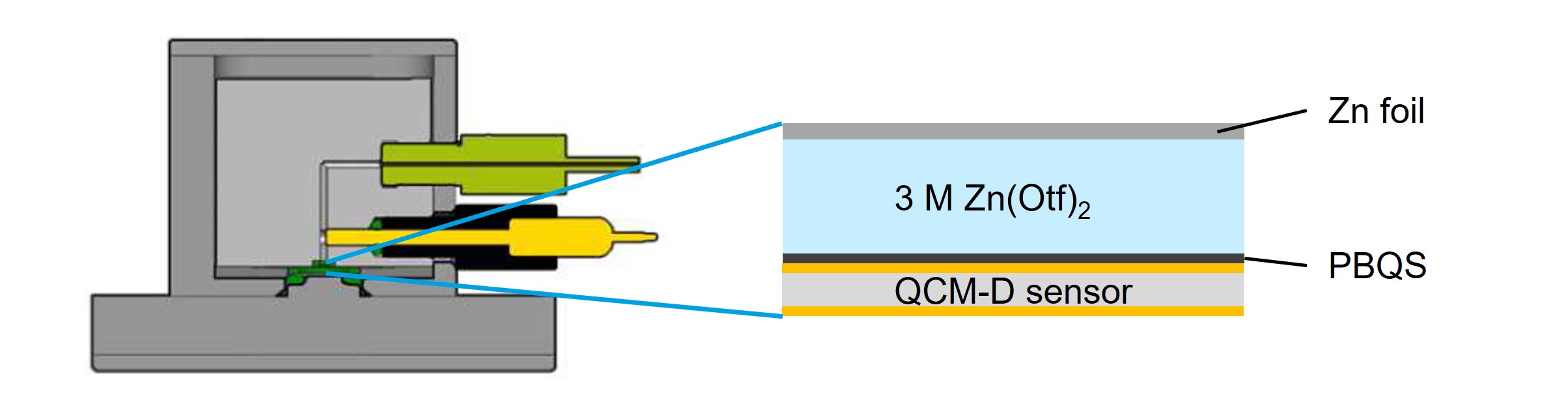
Figure 1. A schematic illustration of the cross section of QSense EQCM-D module showing the electrode configuration and electrolyte used in the study.1
Running multi-harmonic EQCM-D measurements, Δf and ΔD were monitored in two operating conditions:
The collected ∆f and ∆D data was analyzed with QSense software to extract the quantified mass, Fig. 2.
The results from the galvanostatic cycling are shown in Fig 2. Looking at the galvanostatic voltage profile and EQCM-D responses for eleven cycles, Fig. 2A, it is noted that the f3 - change within each cycle is reversible, except for the first two cycles (see ref. 1 for detailed discussion).
From the galvanostatic cycling data, Fig. 2B, the authors conclude that the electrochemical performance is stable, and that this indicates that there is no dissolution of the electrode material candidate, a behavior which is otherwise typically observed for organic electrodes. They also conclude that the specific capacity, 220 mAh·g,−1 which was calculated using the mass of the PBQS, agrees with the capacity measured from coin cells.
The dominant reaction mechanisms are explored in more detail by taking a closer look at the EQCM-D response of a typical cycle, and the authors selected cycle 11, Fig. 2C. The data shows that the mass of the PBQS film increases during the discharge. At the same time, the dissipation decreases, which indicates that the film stiffens. The process is reversible, and during the charge process, the mass decreases and the film becomes softer.
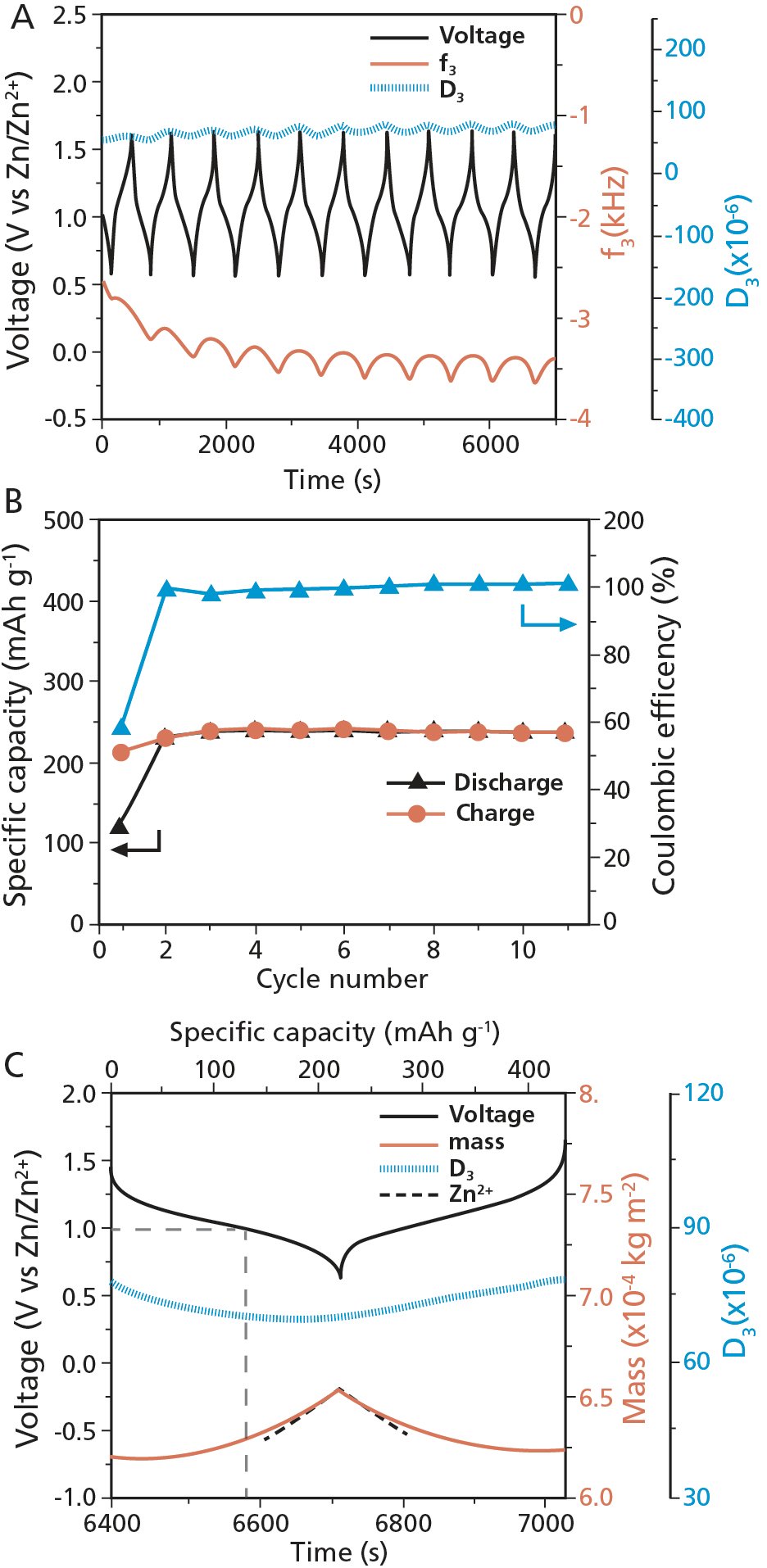
Figure 2. Results from the galvanostatic cycling measurements. A) Voltage profile and EQCM-D response for the PBQS in near-neutral electrolyte for 11 cycles, B) specific capacity and coulombic efficiency C) voltage, dissipation, measured mass calculated from the frequency data, as well as Zn2+ mass calculated based on Faraday’s law.
In the study here presented, the goal was to investigate the charge storage mechanism of a quinone polymer, PBQS, to evaluate the possibility for this material to be used in aqueous zinc ion batteries (AZIBs). Quinones have shown promise to be used as cathode material in aqueous batteries, and as the ion species stored at the cathode is key for the viability of the battery this must be verified.
The analysis was made using combined EQCM-D and FTIR, where EQCM-D provided time resolved information of the changes in mass and viscoelasticity of the cathode material during galvanostatic and cyclic voltammetry cycling. The EQCM-D data showed that the electrode mass increased during discharge and decreased during charge. This information was used to calculate the mass change per charge transfer and revealed that naked Zn2+, rather than its hydrated form, was the cation species stored in the PBQS polymer at voltages < 1V, and that Triflate anions participated in the redox reaction at voltage > 1V.
Based on the study, the authors conclude that quinone compounds have the potential to be used as cathode materials in aqueous zinc-ion batteries and that EQCM-D and FITR is a powerful combination to explore reaction mechanisms of organic electrode materials.
Download the case study to learn more about the user case and how QSense EQCM-D was used in the development of new battery electrode materials
Discover how chronopotentiometry, galvanostatic cycling, and EQCM-D reveal electrochemical mechanisms, battery performance, and material changes.
Webinar introducing the energy transition and key electrochemical energy conversion technologies
Wettability plays a crucial role in the efficiency and performance of PEMFCs as it affects how well the FC components manage water.
Wettability plays a pivotal role in Li-ion battery manufacturing, performance, and safety.
The wetting characteristics of the electrode material play a pivotal role in both the manufacturing and performance of batteries.
Read about how EQCM and EQCM-D are used in battery development and help researchers take battery performance to the next level.
Read about how QSense EQCM-D analysis was used to explore the build-up, evolution, and mechanical properties of the solid electrolyte interphase (SEI).
Calendering, a common compaction process for Li-ion batteries, will significantly impact the pore structure and thus also the wettability of the electrode.
Erik Nilebäck, Ph.D., is a former employee of Biolin Scientific, where he held the position of Senior Application Scientist. Erik has a broad scientific background in research projects and innovation management. He is particularly interested in surface science and earned his Ph.D. in bioscience in 2013, during which he developed biosensing applications focusing on QCM-D.
