Over the past couple of decades, the pharmaceutical industry has increasingly turned its attention away from small molecules and moved towards biological macromolecules, such as monoclonal antibodies (mAbs). Small molecule drugs still dominate the pharmaceutical market, but due to the treatment efficiency for various diseases, biologics, which is today a billion-dollar industry, is on a steady rise and is expected to continue to grow in popularity.1,2 Despite the great promise of these molecules to be able to address diseases where no treatment options currently are available, there are several challenges associated with the development of biopharmaceuticals. Compared to the small-molecule drugs, the biological macromolecules are large and much more complex. They are difficult to manufacture, sensitive to the ambient environment, and impossible to fully characterize. One aspect that is relevant in the context of characterization is that of surface interaction. Biological macromolecules are prone to interact with surfaces, and in doing so, they may adsorb, which could result in loss of concentration and/or particle formation. Over their life span, from manufacturing to storage and administration, the therapeutic molecules will meet many different surfaces, and every interface that they meet after the terminal filtration step presents a risk of reducing the concentration or generating particles. Depending on drug and dose, the consequence may be an unacceptable decrease in concentration or increase of particle formation, posing a risk to product development timelines and a risk of large financial costs such as failing product release specifications during manufacturing. Surface-interaction analysis during development and formulation could provide an indication of challenges that could occur in, or during, manufacturing, storage, and administration. However, methods to screen surface-induced instabilities have not yet been established, which increases the risk of late discovery of such unwanted scenarios.
QSense QCM-D analysis can be used to assess antibody adsorption to gain insight into when and why incompatibilities could occur and to identify ways to mitigate. As a proof of concept, we performed a study that demonstrates the capabilities of the technology to measure antibody adsorption to relevant container closure materials for biologics production with emphasis on pre-filled syringes. We also looked at what effect the added surfactant would have on the adsorption.
The mAb adsorption to four different materials was assessed.
The time-resolved QCM-D data, Fig 1. revealed that adsorption was highest on the metal surface and decreased on silicone oil, plastics, and glass respectively.
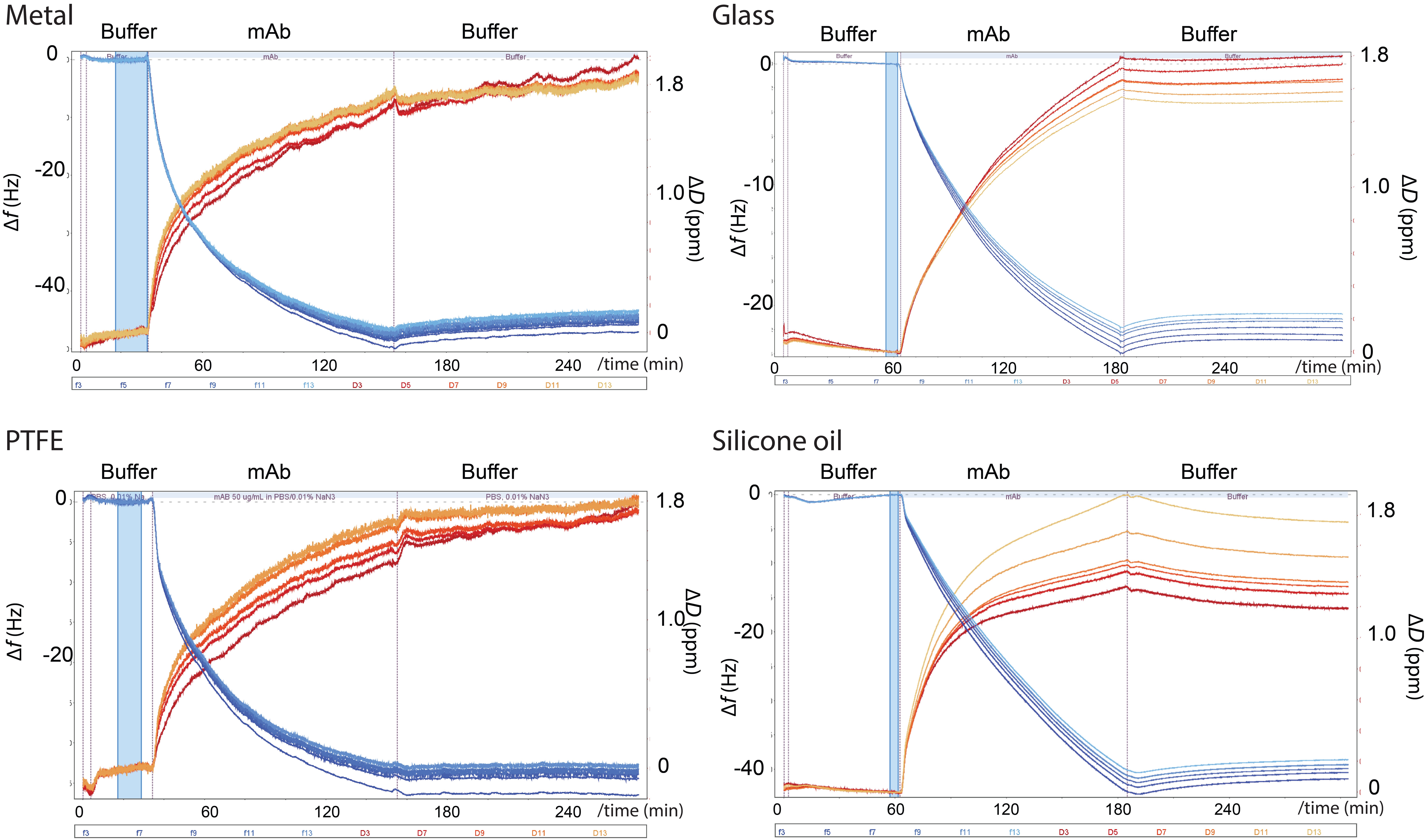
Figure 1. QCM-D raw data, Δf and ΔD, showing time-resolved mAb-surface interaction with the pre-filled syringe mimicking materials steel, glass, plastics, and silicone oil.
Comparing the maximum Δf and ΔD responses and the acoustic ratio, ΔD/Δf, it was noted that:
We also looked at the effect of mAb adsorption to silicon oil and glass when co-formulated with a non-ionic surfactant. The results (data not shown) revealed that the surfactant binds with fast kinetics on silicone oil and lowers the amount of bound mAb by 95%., while on glass the surfactant interaction is weak and the amount of bound mAb increased by 67%.
The pharmaceutical industry's shift towards biological macromolecules, such as monoclonal antibodies, presents challenges due to the complex nature and susceptibility to surface interactions of these molecules. Antibody adsorption could, for example, lead to an unacceptable decrease in concentration and/or an increase in proteinaceous particles. Time-resolved analysis of molecule–surface interaction early on in development can provide valuable insight into the adsorption dynamics of biologics and excipients to materials used in manufacturing, storage, and administration and thereby decrease the risk of late discovery of incompatibilities. In this post, we demonstrated this approach and showed an example of how QSense QCM-D technology can be used to assess antibody and surfactant adsorption to materials used in prefilled syringes.
Download the white paper to learn more about the study
C. Anselmo, et al., Nature Reviews Drug Discovery 2019, 18 (1), 19-40
S. Farid, et al., mAbs 2020, 12 (1), 1754999
Learn how QSense QCM-D reveals protein–surface interactions and adds interface-focused insight to biopharmaceutical formulation and stability work
Read about how molecule-surface interaction processes such as adsorption and desorption can be analyzed with QCM-D.
Learn how QSense QCM-D helps detect and prevent surface-induced instabilities in biologics. Join our webinar for insights and practical examples.
Learn about how QSense analysis can be used to assess adsorption of biologics and excipients to materials used in IV-bags
Read about how QSense QCM-D was used to study antibody fouling on steel surfaces.
Read about how protein adsorption at various surface and solution conditions quickly can be measured
QCM-D is utilized to understand the compatibility and to identify solution and excipient conditions that minimize the adsorption of therapeutic proteins
Learn about how biointerfaces and biomolecular interactions can be studied using QSense® QCM-D and what information these measurements offer.
Erik Nilebäck, Ph.D., is a former employee of Biolin Scientific, where he held the position of Senior Application Scientist. Erik has a broad scientific background in research projects and innovation management. He is particularly interested in surface science and earned his Ph.D. in bioscience in 2013, during which he developed biosensing applications focusing on QCM-D.
