Electrochemistry explained in one single sentence would be “a situation where you can link an electrical potential difference to an identifiable chemical change”, i.e. the potential difference results in a chemical process, reaction, or similar. But there is of course much more to say about electrochemistry. In this blog post, we go into a bit more detail and explain the working principles of electrochemistry and where it is typically used.
One of the first examples of electrochemistry was the Italian physicist Alessandro Volta’s battery stack back in 1800, where he stacked silver and zinc plates with salt brine-soaked cloths in between. This cleverly designed stack generated a potential difference of 1 Volt, a unit named after Alessandro himself. Volta’s battery stack was the first real example of a battery. Later, he developed the concept even further to improve its performance and used the materials copper and zinc instead.
So how could this potential difference be created from a stack of metals? The trick is to utilize and combine the difference in material properties in a clever way.

Figure 1. Alessandro Volta. Italian chemist, physicist, and pioneer of electricity and power. 1745-1827. Antique illustration 1899.
Fig. 2 shows a schematic illustration of a so-called galvanic, or voltaic, cell. This setup consists of a cathode, here a copper rod, on the right-hand side, and an anode, here a zinc rod, on the left-hand side. In between, there is an electrolyte, which creates a closed circuit and enables current to flow.
The resulting potential difference depends on the standard reduction potentials of the materials used in the electrodes, in the case Zn and Cu. The standard reduction potential for Zn to be reduced is -0.76 V and the potential for Cu2+ to be oxidized is 0.34V. In total there is a potential difference of 1.1V which will create a flow of ions; copper ions will flow from solution and create solid copper on the copper rod whereas the zinc will go from the solid zinc rod to zinc ions and into solution. I.e. there will be a flow of electrons from the zinc rod to the copper solution. This concept is the rationale behind the working principle of batteries.
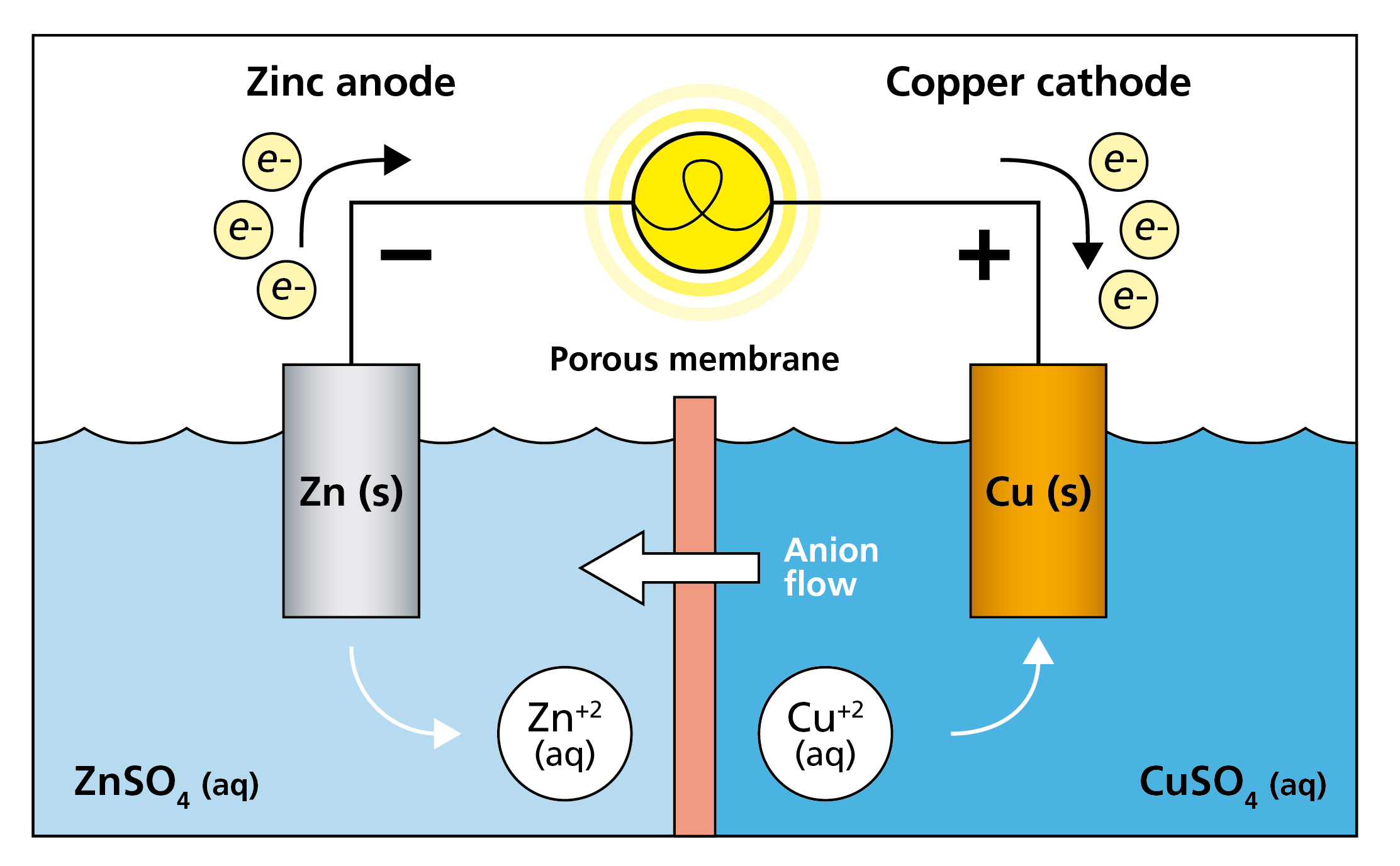 Figure 2. Schematic illustration of a Galvanic cell, also called Voltaic cell
Figure 2. Schematic illustration of a Galvanic cell, also called Voltaic cell
The reaction in the galvanic cell is spontaneous, i.e. the flow of electrons spontaneously occurs when the materials in the cell are combined. If you instead want to drive non-spontaneous reduction-oxidation reactions, so-called redox reactions, this is possible if you apply an external power supply to the setup. Fig. 3, shows a schematic illustration of an electrochemical cell with an external power supply to drive non-spontaneous redox reactions. This setup works very much the same way as the copper-zinc galvanic cell, with the reduction process taking place at the cathode and the oxidation process at the anode, but in this case, however, no electrons will flow unless there is an external power supply driving the flow.
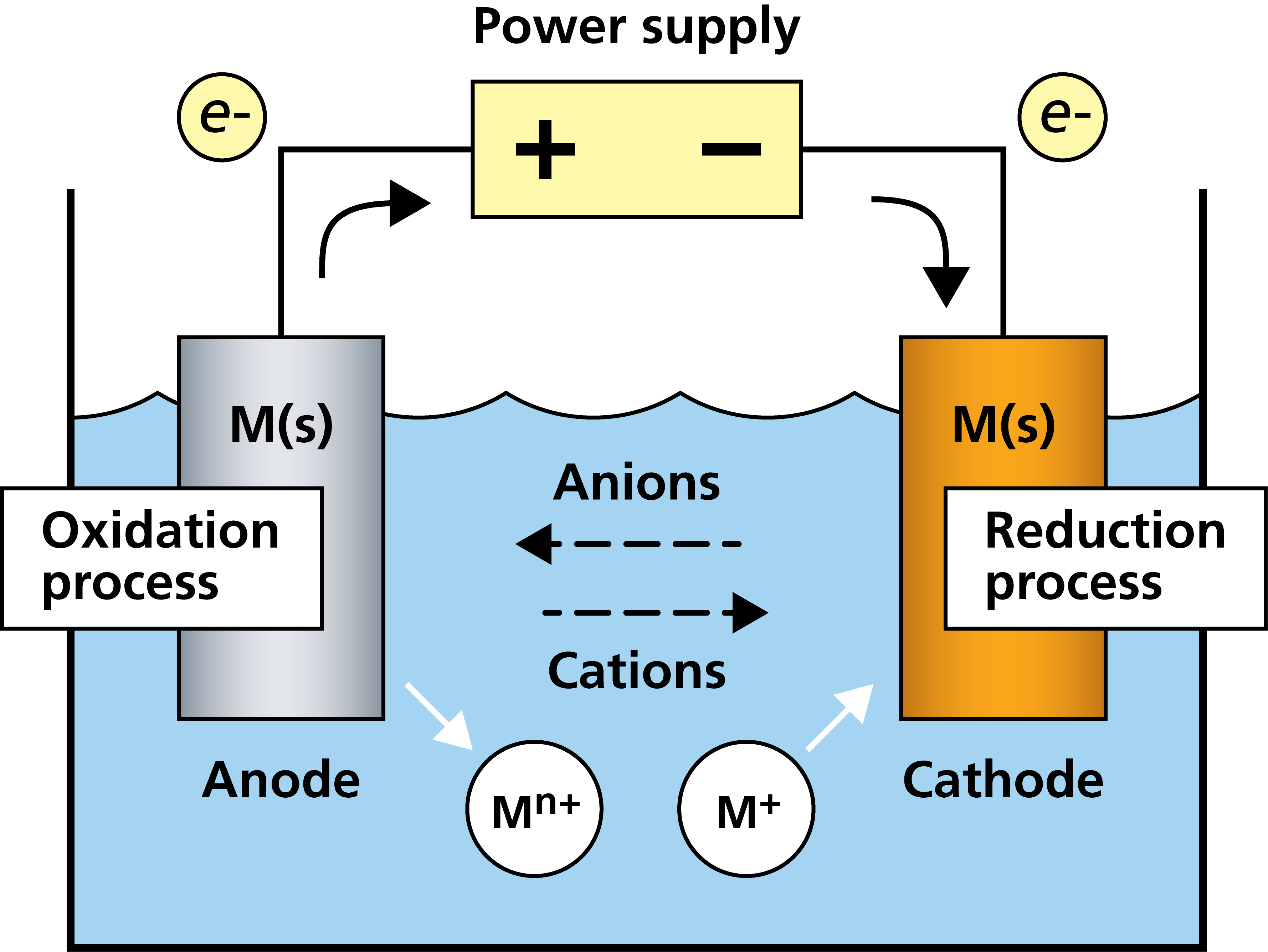
Figure 3. Schematic illustration of an electrochemical cell
To make the setup even more advanced, you could introduce voltage cycling, i.e. you could cycle the cathode potential from positive to negative potential at a certain periodicity. You still have the oxidation-reduction process as in the electric oxidation example, but the potential cycling from positive to negative potential would create a positive and negative current. This approach is called cyclic voltammetry.
The potential cycling will generate a positive and negative current, as illustrated at the bottom of Fig. 4 for an electrochemical cell where solid copper is plated onto a gold-coated sensor from a solution of CuSO4. Starting at the bottom right in the figure, we start with a positive potential and move towards a negative potential, reducing Cu2+ to Cu(s) at the cathode. First, there is no current and then, at a certain potential, you get a negative current that peaks when the Cu2+ to Cu(s) reduction is diffusion-limited. Then we move back from the negative potential minimum towards positive potential and now we will get a positive current when Cu(s) at the cathode is oxidized back to Cu2+. The oxidation peak is highest when all Cu(s) has been oxidized and then rapidly falls down to a current of 0 A
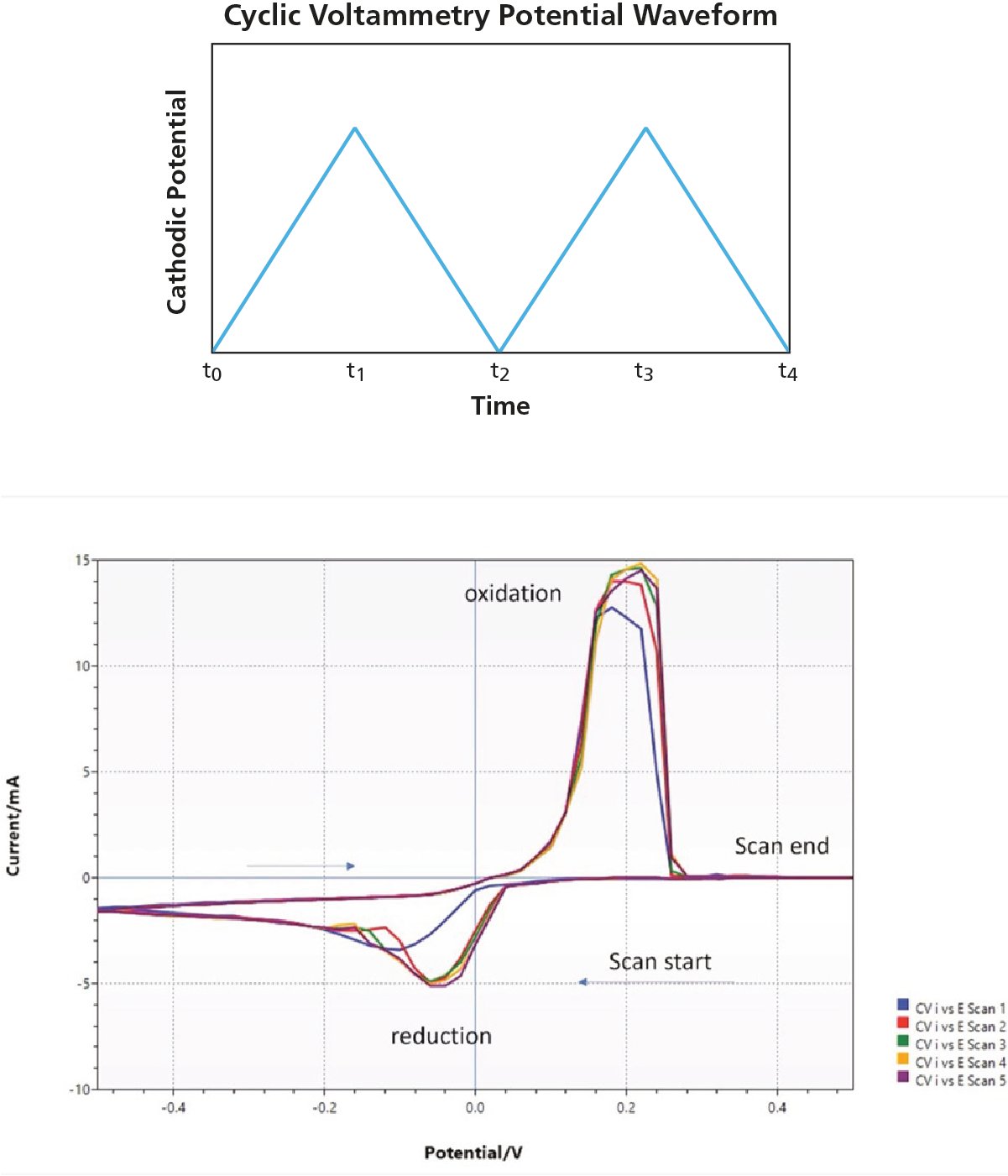
Figure 4. Voltammetry potential waveform (top) and resulting voltammogram (bottom)
So, you will have a scenario of cycling between solid copper and copper ions in solution. This can be monitored with QCM-D, discussed elsewhere.
Electrochemistry is used in a wide variety of application areas, from refining and large-scale metal extraction in electrometallurgy to electrochemistry-based analytical instruments used in life science. Another area that is very relevant in the context of the ongoing global electrification of society is of course that of batteries. There are significant efforts and investments being made in this area. Related to energy storage and production we have fuel cells where you convert chemical energy, for example from H2, to electricity by flow of fuel and oxidizing agent, e.g. O2.
This was a brief introduction to some of the terminology and working principles of electrochemistry. To learn more about electrochemistry and its combination with QCM-D, so-called EQCM-D, please watch the webinar below.
Discover how chronopotentiometry, galvanostatic cycling, and EQCM-D reveal electrochemical mechanisms, battery performance, and material changes.
Discover how amperometric techniques like chronoamperometry and voltammetry reveal real-time interfacial electrochemistry and reaction dynamics
Read about redox reactions, the Nernst equation and what currents can reveal about electrochemical reactions
By integrating QCM-D and electrochemistry into EQCM-D, it is possible to answer questions that neither technique could address alone.
Webinar introducing the energy transition and key electrochemical energy conversion technologies
Read about how EQCM and EQCM-D are used in battery development and help researchers take battery performance to the next level.
Read about how QSense EQCM-D analysis was used to explore the build-up, evolution, and mechanical properties of the solid electrolyte interphase (SEI).
Read about how QSense EQCM-D, Electrochemical Quartz Crystal microbalance with Dissipation monitoring, was used to analyse new battery electrode materials.
Erik Nilebäck, Ph.D., is a former employee of Biolin Scientific, where he held the position of Senior Application Scientist. Erik has a broad scientific background in research projects and innovation management. He is particularly interested in surface science and earned his Ph.D. in bioscience in 2013, during which he developed biosensing applications focusing on QCM-D.
