Surfactants are highly versatile products in the chemical industry, used across various sectors from household detergents to drilling muds, and from food products to pharmaceuticals. The term "surfactant" is derived from "surface active agent." These molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic parts, and they naturally align at the air-water interface. In this alignment, the hydrophobic part extends into the air while the hydrophilic part remains in the water, leading to a reduction in surface or interfacial tension.
Surfactants are amphiphilic molecules with distinct hydrophobic and hydrophilic components. The hydrophobic tail can be a hydrocarbon, fluorocarbon, or siloxane. Surfactants are generally categorized based on their polar head groups, as the hydrophobic tails are usually similar.
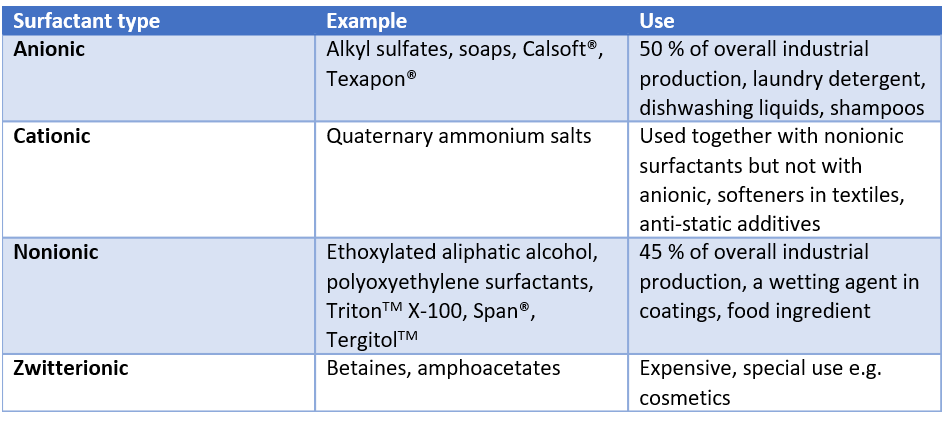
Anionic surfactants are surfactants with a negatively charged head group, making them highly effective at removing dirt and grease. They are widely used in household cleaning products such as laundry detergents, dishwashing liquids, and shampoos due to their excellent foaming and cleaning properties. Anionic surfactants are also utilized in industrial applications, including textile processing and emulsification in agriculture. Common examples of anionic surfactants include Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES), both known for their ability to create rich lathers and effectively break down oils and fats.
Cationic surfactants possess a positively charged head group, which gives them unique properties such as antimicrobial activity and the ability to bind to negatively charged surfaces. These surfactants are commonly used in fabric softeners, hair conditioners, and antistatic agents due to their conditioning effects and ability to reduce static cling. In addition, cationic surfactants are utilized in disinfectants and sanitizers because of their effectiveness in killing bacteria and viruses. Examples of cationic surfactants include benzalkonium chloride and cetyltrimethylammonium bromide, both of which are frequently used in personal care and cleaning products.
Non-ionic surfactants are characterized by their lack of electrical charge in the head group, which makes them particularly useful in applications where compatibility with various substances is important. They are commonly used in stabilizing emulsions, such as oil-in-water and water-in-oil emulsions, and are prevalent in cosmetic products designed for sensitive skin, baby care, and everyday skin care. Additionally, non-ionic surfactants are used in household cleaning products like laundry detergents, toilet bowl cleaners, and dishwashing detergents due to their resistance to water hardness. Examples of non-ionic surfactants include Tween 20 and Triton X-100.
Zwitterionic surfactants contain both positive and negative charges within the same molecule, allowing them to exhibit unique properties such as high solubility and low irritation potential. These surfactants are particularly useful in personal care products like shampoos and body washes, where mildness and compatibility with the skin are important. They are also employed in the formulation of pharmaceuticals and cosmetics due to their ability to stabilize proteins and emulsions. Examples of zwitterionic surfactants include cocamidopropyl betaine and sulfobetaine, both of which are valued for their gentle cleansing and foaming capabilities.
Due to their amphiphilic nature, surfactants are absorbed in the air-water or oil-water interface. At these interfaces, surfactants align such that the hydrophobic part is in the air (or oil) and the hydrophilic part is in the water.
Focusing on the air-water interface, the strong cohesive forces between water molecules result in high surface tension. When surfactants are absorbed, they disrupt these interactions. The intermolecular forces between surfactant and water molecules are weaker than those between water molecules, leading to a reduction in surface tension. At high surfactant concentrations, micelles form, with the concentration at which this occurs known as the critical micelle concentration.

The primary function of surfactants is to reduce surface and interfacial tension and stabilize interfaces. Without surfactants, tasks like washing laundry would be challenging, and products like mayonnaise and ice cream might not exist. Therefore, optimizing surfactants for various applications is crucial, with surface and interfacial tension measurements playing a key role in this process.
If you would like to learn about the different roles of surfactants in industrial processes and products, please watch our recorded webinar below.
This blog post was originally published on the 26th of June, 2018, and has since been updated for completeness.
A wetting agent is a surface-active molecule used to reduce the surface tension of water.
Surface tension plays an important role in Li-ion battery slurry optimization.
Surface tension plays an important role in the electroplating solution.
When measuring contact angles or making surface tension measurements with a pendant drop, selecting the correct tip or needle for your liquid is crucial.
The surface tension of water is about 72 mN/m at room temperature which is one of the highest surface tension for liquid.
Surface tension is a quantitative measure that can be correlated with a solution’s ability to remove dirt.
Surface tension and wettability are important physical properties that play a significant role in the effectiveness of agrochemicals.
Explains three different methods to measure surface tension.
