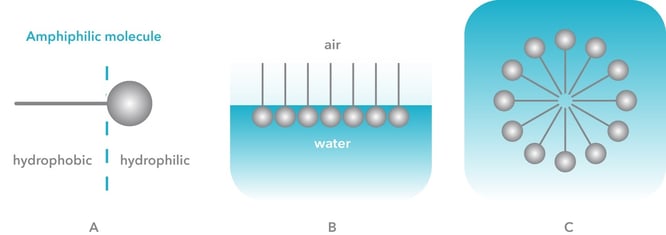
To understand critical micelle concentration, or CMC for short, we need first to understand surfactants. Surfactants are amphiphilic molecules that have hydrophilic and hydrophobic parts (figure 1A). When surfactants are added to water, they orient themselves at the air-water interface so that the hydrophilic part is in water and hydrophobic part in air (figure 1B). Another energetically favorable structure is a micelle (figure 1C), where hydrophobic parts are pointing inwards the spherical structure protected by the hydrophilic outer shell.
The main reason to add surfactants to a solution is to decrease surface tension. The surface tension of water is high due to hydrogen bonding between water molecules. When surfactants are added, they will break those bonds by penetrating at the air-water interface. This will lower the surface tension of water. There are several reasons why the surface tension should be lowered, but maybe the most practical example comes from laundry detergents. When washing laundry, water should penetrate in between the fabric fibers. Due to the high surface tension of water, it is by itself a poor cleaning agent, and thus laundry detergents containing surfactants are added.
Let’s continue with the same laundry detergent example. Landry detergents contain several ingredients, surfactants being one of the most important ones. Due to both economic and environmental reasons, the amount of surfactant should be minimized. As surfactants are added into the detergent mixture, the surface tension will decrease. However, at some point, the surface becomes saturated with surfactant molecules, and micelles start to form (figure 2). This point is defined as critical micelle concentration. After this point, the addition of surfactants will no longer affect the surface tension and is therefore unnecessary. Critical micelle concentration can be defined by measuring surface tension as a function of surfactant concentration.
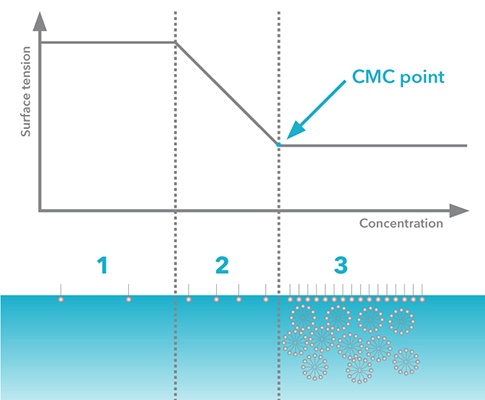
Figure 2. Surface tension as a function of surfactant concentration (logarithmic scale)
To read more about critical micelle concentration, please download the overview through the link below.
A wetting agent is a surface-active molecule used to reduce the surface tension of water.
The term surfactant comes from the word surface active agent. At the interface, they align themselves so that the hydrophobic part is in the air and the hydrophilic part is in water. This will cause a decrease in surface or interfacial tensions.
Surface tension plays an important role in Li-ion battery slurry optimization.
Surface tension plays an important role in the electroplating solution.
When measuring contact angles or making surface tension measurements with a pendant drop, selecting the correct tip or needle for your liquid is crucial.
The surface tension of water is about 72 mN/m at room temperature which is one of the highest surface tension for liquid.
