.jpg?height=480&name=C311-300%20(copy).jpg)
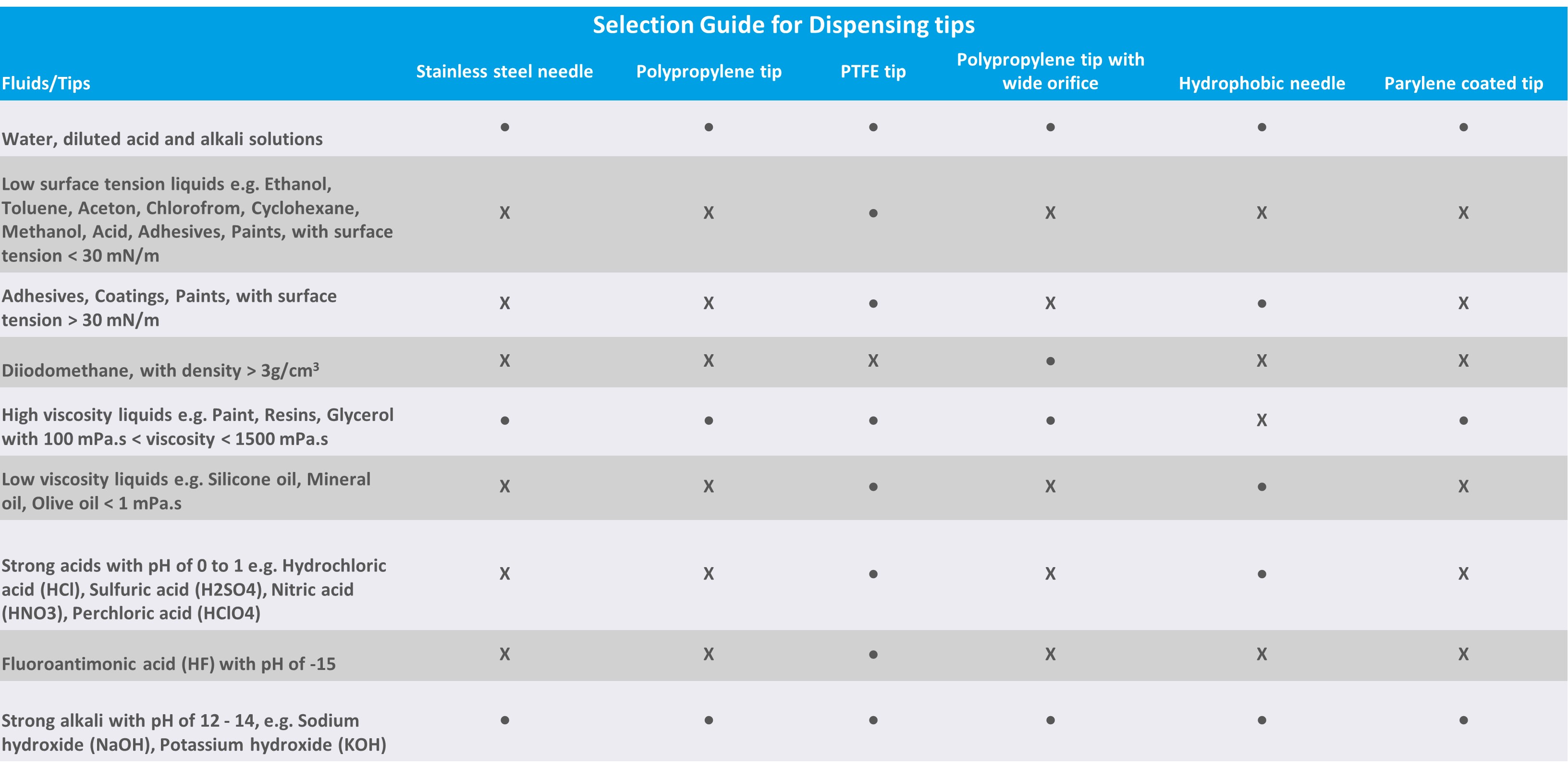
When measuring contact angles or making surface tension measurements with a pendant drop, selecting the correct tip or needle for your liquid is crucial. If you have ever done measurements with low surface tension liquids, you have probably noticed that it will easily creep up the side of the tip. Then on the other hand, diiodomethane, which is often used for surface free energy calculation is problematic not because of its low surface tension but because of its high density. Chemical compatibility and viscosity of the liquids should, of course, also be considered. In this blog post, we elaborate on the liquid properties that affect the drop formation as well as give general guidelines on which tip to choose for your liquid.
The low surface tension of the liquid will make the drop formation with standard polypropylene tips or stainless-steel needles problematic. This is because liquids with surface tension values lower than 30 mN/m will easily wet the material, leading to liquid rising on the side of the tip. In the picture below, ethanol with a surface tension lower than 20 mN/m has been dispensed with polypropylene (left) and PFA tips (right). The PFA tip is suitable for dispensing ethanol whereas polypropylene is not.

Although most of the liquids have densities lower than water there are still some commonly used ones with so high density that it needs to be taken into account when dispensing. Di-iodomethane is a good example of such a liquid as it is often used as a probe liquid in surface free energy calculations. The surface tension of di-iodomethane is quite high which supports relatively big drops. However, as the density is also high, the maximum volume a tip can hold is less than would be expected by looking at surface tension alone. To make the dispensing of di-iodomethane more reliable, a tip with a larger orifice size should be used.
Chemical compatibility and viscosity are also important properties to consider. The tip material should always be selected so that it is chemically compatible with the liquid measured. In principle, this means that if you are measuring very harsh acids the PFA tips should be chosen. The same applies to some organic solvents like toluene.
If the viscosity of the liquid is high, the orifice size of the tip or needle needs to be big to allow liquid to go through it.
A wetting agent is a surface-active molecule used to reduce the surface tension of water.
The term surfactant comes from the word surface active agent. At the interface, they align themselves so that the hydrophobic part is in the air and the hydrophilic part is in water. This will cause a decrease in surface or interfacial tensions.
Surface tension plays an important role in Li-ion battery slurry optimization.
Surface tension plays an important role in the electroplating solution.
The surface tension of water is about 72 mN/m at room temperature which is one of the highest surface tension for liquid.
Surface tension is a quantitative measure that can be correlated with a solution’s ability to remove dirt.
Surface tension and wettability are important physical properties that play a significant role in the effectiveness of agrochemicals.
Explains three different methods to measure surface tension.
