Electroplating is a technique that deposits a thin layer of metal onto a conductive surface using an electrical current. This process involves a bath, or solution, containing ions of the metal being deposited, and an electrical circuit comprising the object to be plated (the cathode), a source of the plating metal (the anode), and an electrolyte solution. Surface tension plays an important role in the electroplating solution.
Surface tension is a property of liquids that arises from the cohesive forces between the molecules at the surface of the liquid. Molecules within a liquid experience attractive forces in all directions due to intermolecular forces. However, at the surface, these molecules don't have neighboring molecules on one side, resulting in a net inward force, creating a phenomenon known as surface tension.
Different liquids have varying surface tensions based on their molecular properties.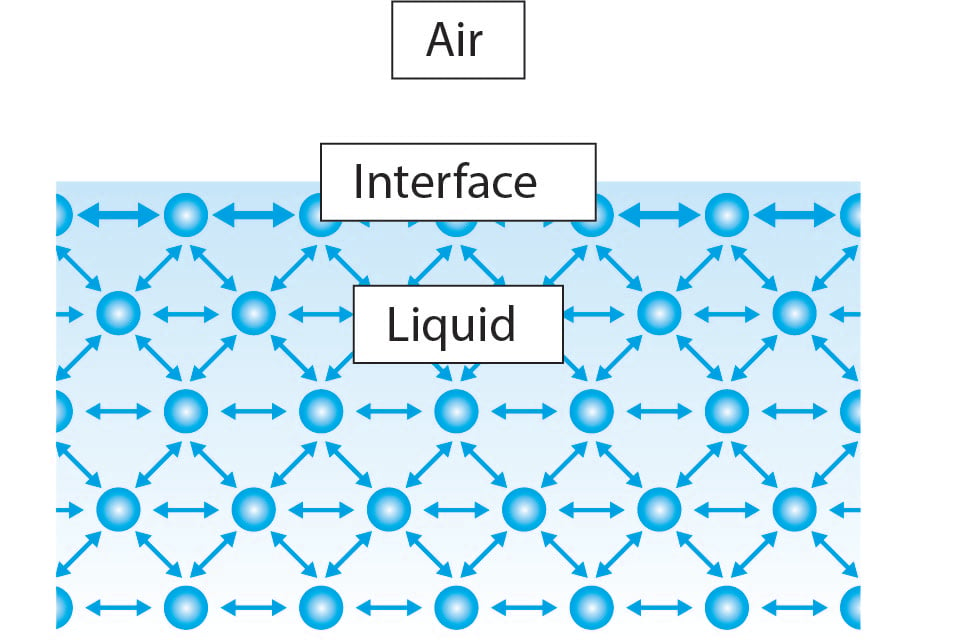 For example, water has relatively high surface tension due to its strong hydrogen bonding between molecules, while liquids like alcohol or oil have lower surface tensions due to weaker intermolecular forces.
For example, water has relatively high surface tension due to its strong hydrogen bonding between molecules, while liquids like alcohol or oil have lower surface tensions due to weaker intermolecular forces.
In the electroplating process, if the surface tension of the plating solution is too high, that will lead to insufficient gap-filling. This will result in small voids not being coated with the metal. If surface tension is reduced, the gap-filling is excellent but the solution is prone foam. The small air bubbles formed will cause voids on the surface of the metal. The optimum surface tension of the electroplating solution is typically around 30 to 35 mN/m.
Method 306B describes how the surface tension measurement of the chromium electroplating solution should be conducted. When using a force tensiometer, an additional standard ASTM D 1331 should be followed. The surface tension of the electroplating bath should be frequently followed. During the first 40 hours of tank operation, surface tension should be measured every 4 hours. If the surface tension has been within the set limits, the interval can be reduced to every 8 hours. If the surface tension is still within limits after the second 40 hours of operation, the sampling can be reduced to every 40 hours. Once the surface tension exceeds 45 mN/m, the time interval should be reverted to the original monitoring schedule of once every 4 hours.
If you would like to learn more about surface tension and its measurement techniques, watch the webinar through the link below.
A wetting agent is a surface-active molecule used to reduce the surface tension of water.
The term surfactant comes from the word surface active agent. At the interface, they align themselves so that the hydrophobic part is in the air and the hydrophilic part is in water. This will cause a decrease in surface or interfacial tensions.
Surface tension plays an important role in Li-ion battery slurry optimization.
When measuring contact angles or making surface tension measurements with a pendant drop, selecting the correct tip or needle for your liquid is crucial.
The surface tension of water is about 72 mN/m at room temperature which is one of the highest surface tension for liquid.
Surface tension is a quantitative measure that can be correlated with a solution’s ability to remove dirt.
Surface tension and wettability are important physical properties that play a significant role in the effectiveness of agrochemicals.
Explains three different methods to measure surface tension.
