
Water exhibits a remarkable surface tension, measuring approximately 72 mN/m at room temperature, which places it among the liquids with the highest surface tension. However, there is one exceptional liquid that surpasses water in this regard: mercury, a unique liquid metal boasting an astonishing surface tension of nearly 500 mN/m. The reasons behind mercury's unusually high surface tension will become evident as we delve into the following discussion.
Surface tension originates from the cohesive interactions transpiring between molecules within a liquid. Within the bulk of the liquid, each molecule finds itself flanked by neighboring molecules on all sides. Consequently, the intermolecular forces act in a balanced manner, exerting equal pulls from all directions, thereby resulting in a net force of zero. Conversely, at the liquid's interface, molecules find themselves with only half the number of neighboring molecules compared to the bulk. Consequently, these molecules form stronger associations with their adjacent counterparts, leading to an inward net force exerted upon the liquid. This force effectively resists the rupture of the liquid's surface and is commonly referred to as surface tension.
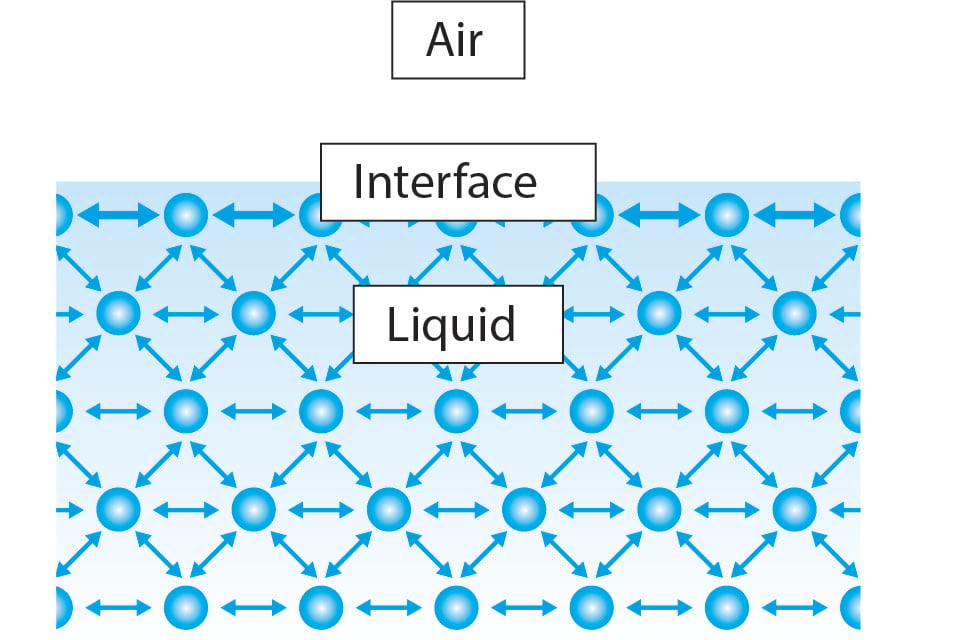
Understanding this explanation, it becomes apparent that all liquids possess this property to some degree. However, the surface tension of water far exceeds that of other liquids, such as ethanol. To comprehend this distinction, we must consider the nature of the bonds existing between water molecules. As previously mentioned, the cohesive forces between molecules are responsible for surface tension. The strength of these cohesive forces directly correlates with the magnitude of surface tension. In the case of water, each water molecule consists of two hydrogen atoms bonded to an oxygen atom through covalent bonding. Due to the high electronegativity of oxygen, a substantial portion of the negative charge is localized on its side, while hydrogen bears a comparatively positive charge. Consequently, an electrostatic attraction occurs between the hydrogen atom in one water molecule and the oxygen atom in another. These bonds are referred to as hydrogen bonds, which engender robust cohesive forces among water molecules, ultimately resulting in the high surface tension observed in water.
Returning to the initial mention of mercury, we can now elucidate the reasons behind its exceptional surface tension. Since mercury is a metal, the bonds connecting its molecules are metallic bonds, considerably stronger than hydrogen bonds. Consequently, the cohesive forces between mercury molecules are significantly intensified, yielding an extraordinarily high surface tension.
In conclusion, the surface tension of water and mercury, despite their distinct magnitudes, arises from the cohesive interactions between their respective molecules. Water's surface tension is predominantly a consequence of the potent hydrogen bonds formed between its molecules, while mercury's surface tension is attributable to the substantially stronger metallic bonds exhibited by its molecular structure.
To learn more about surface tension and how to measure it, please download the white paper below.
This blog post was originally posted on the 4th of August 2020 and has since been updated for increased clarity
A wetting agent is a surface-active molecule used to reduce the surface tension of water.
The term surfactant comes from the word surface active agent. At the interface, they align themselves so that the hydrophobic part is in the air and the hydrophilic part is in water. This will cause a decrease in surface or interfacial tensions.
Surface tension plays an important role in Li-ion battery slurry optimization.
Surface tension plays an important role in the electroplating solution.
When measuring contact angles or making surface tension measurements with a pendant drop, selecting the correct tip or needle for your liquid is crucial.
Surface tension is a quantitative measure that can be correlated with a solution’s ability to remove dirt.
Surface tension and wettability are important physical properties that play a significant role in the effectiveness of agrochemicals.
Explains three different methods to measure surface tension.
