
A stable solid electrolyte interphase (SEI) is key to reversible and stable cycling of a battery. Ideally, this interfacial layer, which forms on the battery anode through electrolyte decomposition during battery charge and discharge cycles, should be mechanically robust, electrically insulating and ionically conductive. In this post, we describe a user case where QSense EQCM-D, Electrochemical Quartz Crystal microbalance with Dissipation monitoring, was used to investigate SEI-formation. The analysis was used to explore how the electrolyte affects the build-up, evolution, and the mechanical properties of the formed interfacial layer.
QSense EQCM-D, Electrochemical Quartz Crystal microbalance with Dissipation monitoring, is a time-resolved surface sensitive technology which is used to characterize molecular interactions and reactions at surfaces and interfaces while monitoring electrical properties through electrochemical measurements. These interfacial processes can be monitored with nano-level sensitivity via two parameters, frequency (∆f) and energy dissipation (∆D) at multiple harmonics, so called multi-harmonic EQCM-D, where the frequency relates to mass changes at the surface and the dissipation relates to the softness or viscoelasticity of the surface-adhering layer. Monitoring changes in mass and viscoelasticity, the method can provide quantitative information on interfacial processes such as the formation, evolution, and mechanical properties of SEIs in different solvents and in the presence of different additives.
Over the years, significant efforts have been made to reveal the conditions at which optimal SEI properties are achieved. In this case study,1 the researchers wanted to understand how the SEI structure, composition and mechanical properties evolve on an active electrode surface in different electrolytes.
Specifically, the goal was to answer the following questions:
To answer the questions above, the researchers used QSense EQCM-D to monitor and analyze the SEI build-up and mechanical properties in three different electrolytes.
The electrolytes used all contained 1.0 mol/L LiClO4, and were:
A two-electrode cell was used, and the electrode configuration was as follows:
Multi-harmonic EQCM-D measurements were performed from open circuit voltage (OCV) to 0V to form SEI:s on the copper coated sensor surfaces, i.e. the WE, in the three different electrolytes. The discharge from OCV to 0V was at a current density of 0.1 mA/cm2.
The EQCM-D analysis revealed several insights on the SEI build-up process and the evolution of the mechanical properties of the formed layers in the three different electrolytes.
Looking at the Δf and ΔD vs discharge time for the PC-, EC/DEC-, and TEGDME-based electrolytes, Fig. 1, the researchers concluded the following
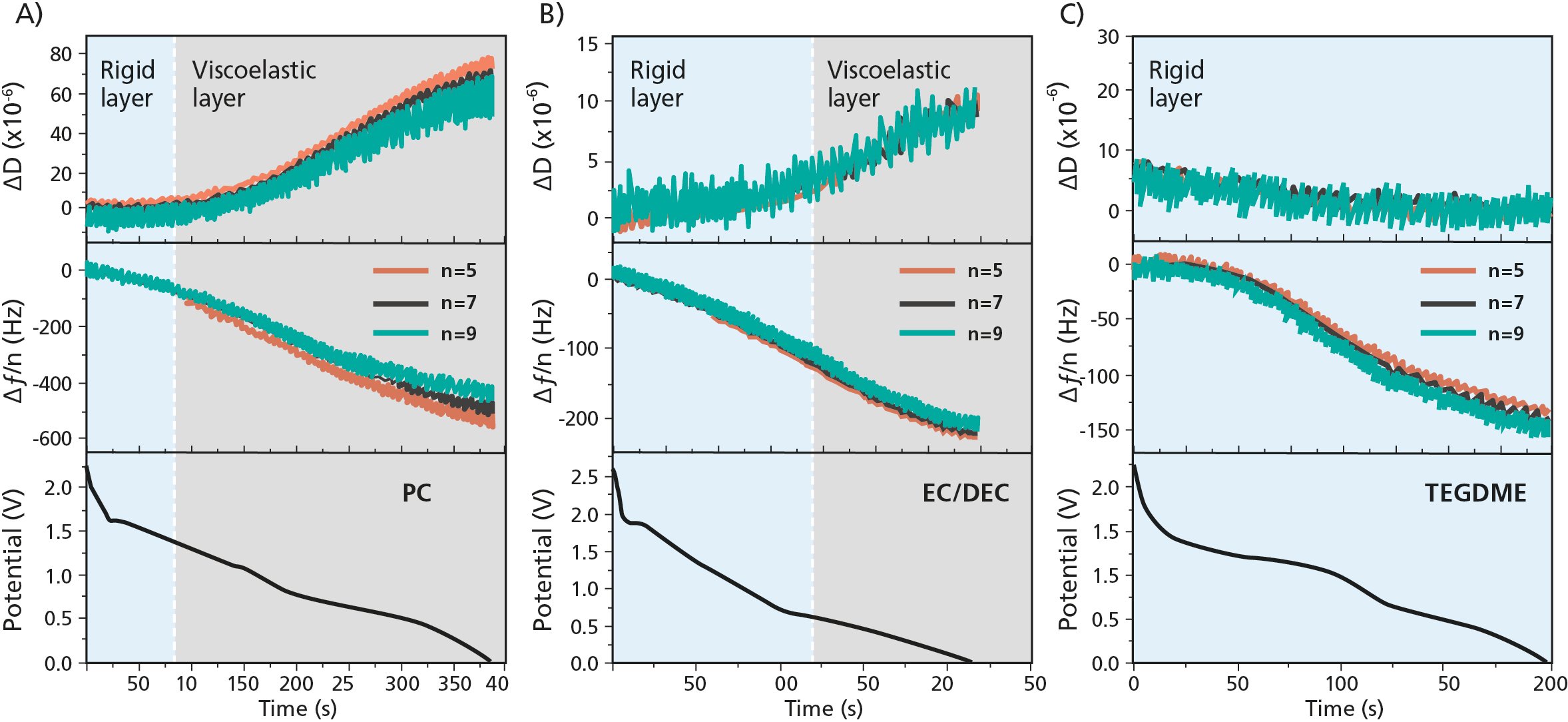
Figure 1. EQCM-D measurements of the SEI build-up process showing Δf and ΔD for three harmonics, n = 5, 7, and 9, and the working electrode potential vs discharge time for the A) PC-, B) EC/DEC-, and C) TEGDME-based electrolytes.
Using the QCM-D data, the researchers quantified the mass and the viscoelastic properties of the layers formed at the WE in the three different electrolytes, PC-, EC/DEC- and TEGDME. The mass of the SEI:s were 20.9 μg/cm2, 4.7 μg/cm2, and 2.4 μg/cm2 in the PC-, EC/DEC- and TEGDME – based electrolytes respectively. The researchers also calculated the mass change per mole of electron transfer (mpe) to extract information on what species that were generated at different voltages in the three cases.
Viscoelastic modelling of the SEI:s formed in the ether- based electrolytes revealed that the shear modulus decreases, and the viscosity increases during the build-up in both PC and EC/DEC- electrolytes. This indicates that soft layers are formed at the electrode/electrolyte interface. It is also noted that the shear modulus is higher for the SEI in the EC/DEC based electrolyte that in the PC based one, which indicates that the formed SEI is more rigid.
Stable SEI:s have been declared critical for reliable operation of Li-ion batteries and there are ongoing efforts to increase the understanding on how to achieve SEI:s to support optimal battery performance. Even though efforts have been made to elucidate how solvents and other components affect the formation, evolution and properties of the SEI:s, however, there still is a lack of understanding in this area. In the study here presented, the researchers wanted to fill some of this knowledge gap. Using EQCM-D analysis, they investigated the SEI build-up process and mechanical properties, and how these depend on the electrolyte composition. Three different electrolytes were explored, a propylene carbonate (PC)-based electrolyte, an ethylene carbonate/diethyl carbonate (EC/DEC)-based electrolyte, and a tetraethylene glycol dimethyl ether (TEGDME)-based electrolyte. The time-resolved EQCM-D measurements revealed several key findings. The analysis showed that the SEI build-up process vary greatly between the different electrolytes, and so do the mechanical properties and mass of the SEI:s formed. Based on the study, the authors conclude that in situ EQCM-D helps the understanding of SEI formation and its stability, and that the technology can inspire to new ideas for SEI and electrolyte design in the future.1
Download the case study to learn more about the user case and how QSense EQCM-D was used to investigate SEI formation.
Discover how chronopotentiometry, galvanostatic cycling, and EQCM-D reveal electrochemical mechanisms, battery performance, and material changes.
Webinar introducing the energy transition and key electrochemical energy conversion technologies
Wettability plays a crucial role in the efficiency and performance of PEMFCs as it affects how well the FC components manage water.
Wettability plays a pivotal role in Li-ion battery manufacturing, performance, and safety.
The wetting characteristics of the electrode material play a pivotal role in both the manufacturing and performance of batteries.
Read about how EQCM and EQCM-D are used in battery development and help researchers take battery performance to the next level.
Read about how QSense EQCM-D, Electrochemical Quartz Crystal microbalance with Dissipation monitoring, was used to analyse new battery electrode materials.
Calendering, a common compaction process for Li-ion batteries, will significantly impact the pore structure and thus also the wettability of the electrode.
Erik Nilebäck, Ph.D., is a former employee of Biolin Scientific, where he held the position of Senior Application Scientist. Erik has a broad scientific background in research projects and innovation management. He is particularly interested in surface science and earned his Ph.D. in bioscience in 2013, during which he developed biosensing applications focusing on QCM-D.
