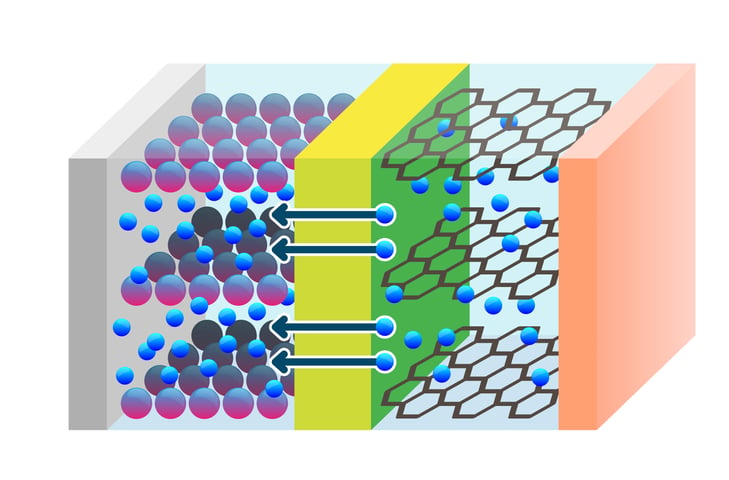
Calendering is a common compaction process for lithium-ion battery electrodes. The purpose of calendering is to reduce the porosity of the electrode which improves the particle contact and thus enhances the energy density of the battery. Calendering will significantly impact the pore structure and thus also the wettability of the electrode.
Wettability of the electrode material with the electrolyte solution is one of the challenges in the development of high-performance lithium-ion batteries. Insufficient electrolyte wetting of porous electrodes leads to irregular reactions in the electrodes and unstable formation of the solid-electrolyte interface film. This can deteriorate the cell performance and cause poor cycle life. In addition, incomplete wetting enables the dendrite formation of lithium metal, which causes severe safety issues. Unwetted active material will also lead to underutilization of electrode capacity and increase electrode resistance.
Electrolyte wetting to the electrode can be studied by measuring the wetting rate with a force tensiometer. The measurement is based on the so-called Washburn method where the porous sample is immersed into the liquid and a highly sensitive balance is used to record the mass uptake as a function of time.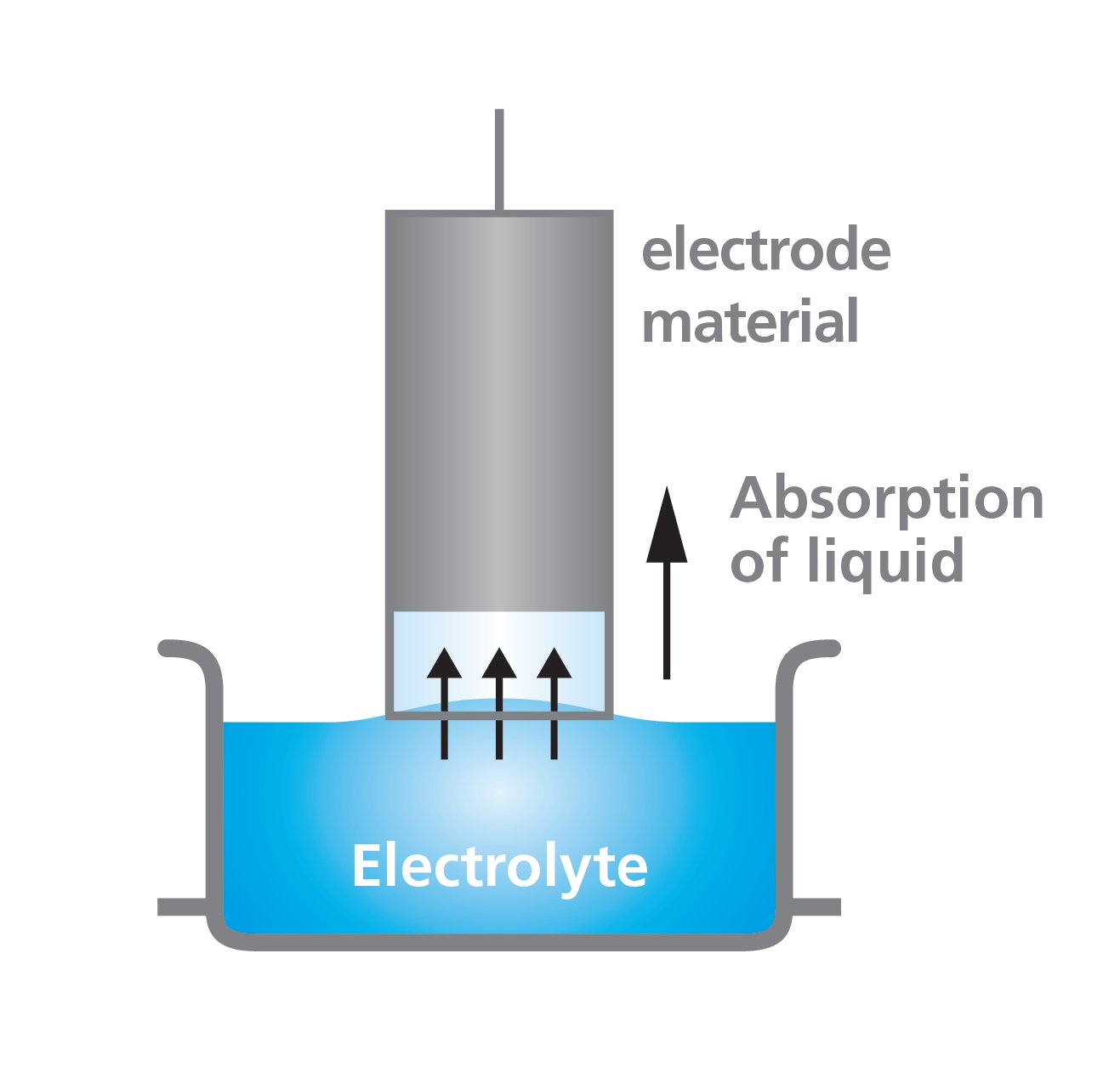
The method has been used to determine the effect of calendering on electrode wettability in lithium-ion batteries. The electrolyte uptake to electrode films of different thicknesses due to the calendering process was measured. The mass uptake as a function of time is recorded.
Moderate calendering has been shown to improve the wettability of the electrode material due to the alignment of the particles and the increase in divergence within the pore network. If calendering is done beyond the optimum level, the wettability of the electrode will decrease as the pore diameter becomes smaller and the porosity is reduced.
Calendering process changes the surface structure of the electrode. Surface roughness has a clear effect on wettability since surface roughness enhances the effect of surface chemistry. If the surface is chemically hydrophilic, the roughness will make the surface even more hydrophilic. For this reason, measuring surface roughness as well as the contact angle can give more insight into the wettability of the surface.
Learn about the effect of surface roughness and wettability on biocompatibility of biomaterials and medical devices.
This blog post describes the importance of fiber diameter on the contact angle measurements of fibers with Wilhelmy method
Standard contact angle measurement considers the surface's chemical properties. The influence of surface roughness is added by utilizing the Wenzel equation.
Wettability is crucial in biomedical applications as it affects protein adsorption, cell adhesion, blood coagulation, and bacterial colonization.
Liquids’ ability to wet a solid surface has widespread importance in many everyday products and industrial processes.
Membrane wettability is a key property to ensure success of membrane distillation process
Hydrophobic surface properties are needed in contact with the food product while hydrophilicity is needed when printing on food packaging.
Wettability is pivotal in pharmaceutical dosage form manufacturing as well as in drug efficacy.
Understanding the wettability of membranes is essential for optimizing these processes and achieving desired separation outcomes.
