
The wetting characteristics of the electrode material play a pivotal role in both the manufacturing and performance of batteries. In the case of Li-ion batteries, the electrode's wetting behavior is directly linked to the battery's capacity and its ability to discharge at high rates. Inadequate wetting can result in suboptimal utilization of the electrode's capacity, heightened resistance, and potential safety issues. Consequently, assessing the wetting properties of the electrode material with respect to the electrolyte is a crucial step in the development of new electrode or electrolyte materials. The force tensiometer serves as an invaluable tool in this regard, facilitating the measurement of both the surface tension of the electrolyte and adsorption studies, both of which significantly influence the overall wetting characteristics.
Li-ion batteries are composed of a cathode and anode, an electrolyte solution, and a separator. The cathode side features an aluminum electrode substrate, while the anode side utilizes copper.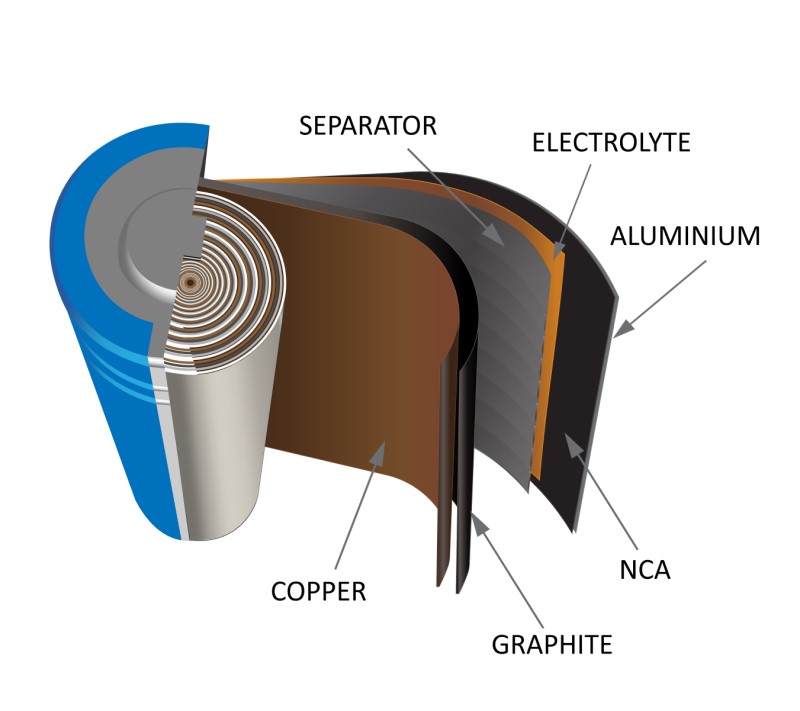 Both substrates are coated with a slurry containing active material. Commonly, the cathode side's active material is a metal oxide like lithium nickel cobalt aluminum oxide (NCA) or lithium nickel manganese cobalt oxide (NMC), each offering distinct levels of energy density, thermal stability, and cost-effectiveness. The anode's active material typically includes graphite, silicon, or a combination of both.
Both substrates are coated with a slurry containing active material. Commonly, the cathode side's active material is a metal oxide like lithium nickel cobalt aluminum oxide (NCA) or lithium nickel manganese cobalt oxide (NMC), each offering distinct levels of energy density, thermal stability, and cost-effectiveness. The anode's active material typically includes graphite, silicon, or a combination of both.
The manufacturing cost of battery cells constitutes a significant portion of the overall battery cost. Furthermore, the materials and metals employed in cathode manufacturing contribute to 30–40% of lithium battery cell costs, whereas anode materials represent approximately 10–15%. Therefore, material selection is crucial for cost-effective production.
The cathode and anode are assembled with the separator material, and the electrolyte solution is injected after stacking. This step, crucial in terms of wettability, marks a pivotal stage in the battery manufacturing process.
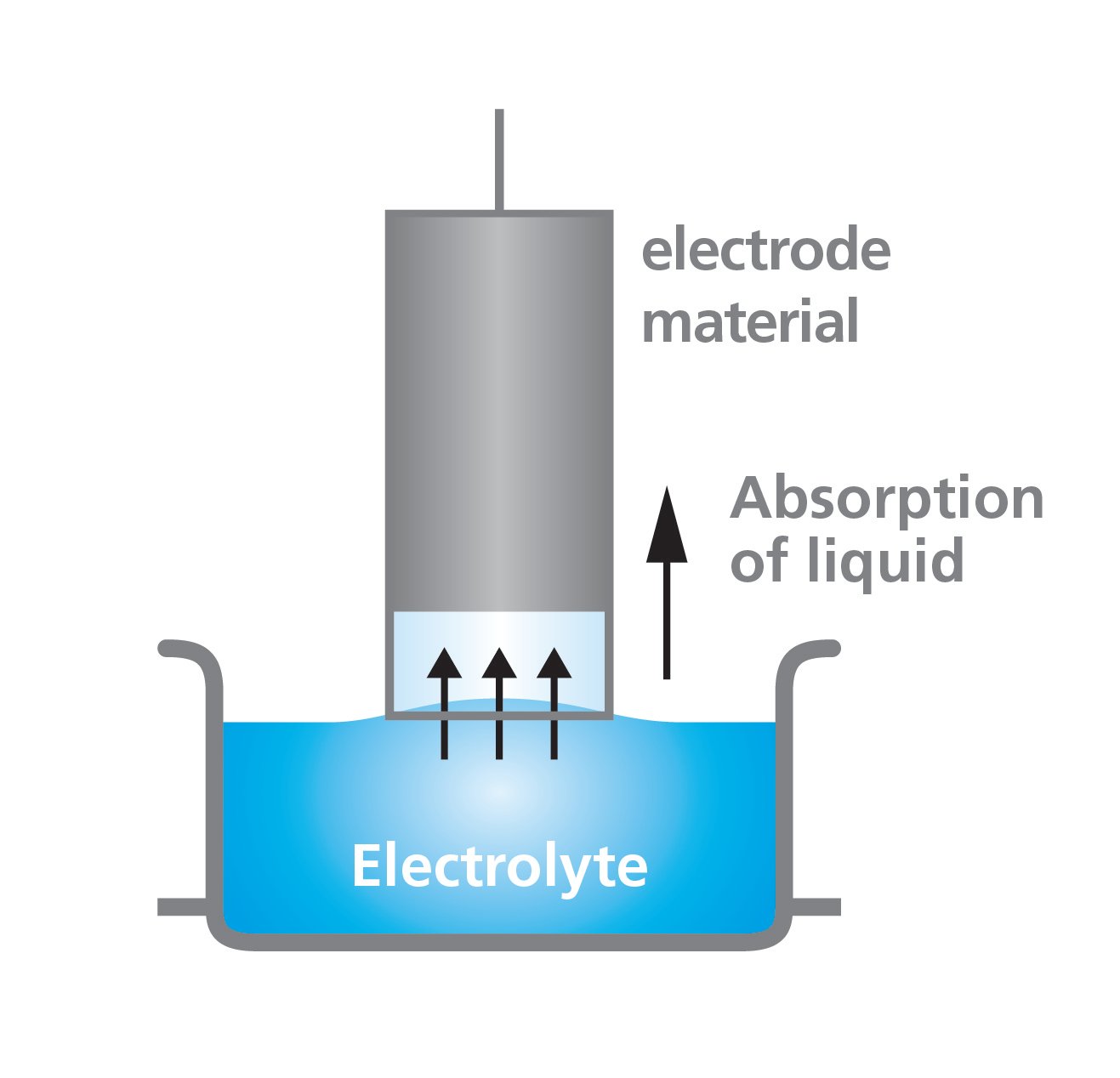 The wettability of the electrode material against the electrolyte can be studied with the force tensiometer. In practice, a piece of electrode is hung on a highly sensitive balance. The electrolyte solution is placed in the beaker. The electrode material is slightly immersed into the electrolyte solution and the mass uptake is recorded. The electrode substrate should be coated with the same slurry on both sides to allow adsorption measurements. To hear more about adsorption measurements, please sign up for the webinar through the link below.
The wettability of the electrode material against the electrolyte can be studied with the force tensiometer. In practice, a piece of electrode is hung on a highly sensitive balance. The electrolyte solution is placed in the beaker. The electrode material is slightly immersed into the electrolyte solution and the mass uptake is recorded. The electrode substrate should be coated with the same slurry on both sides to allow adsorption measurements. To hear more about adsorption measurements, please sign up for the webinar through the link below.
Discover how chronopotentiometry, galvanostatic cycling, and EQCM-D reveal electrochemical mechanisms, battery performance, and material changes.
Webinar introducing the energy transition and key electrochemical energy conversion technologies
Wettability plays a crucial role in the efficiency and performance of PEMFCs as it affects how well the FC components manage water.
Wettability plays a pivotal role in Li-ion battery manufacturing, performance, and safety.
Read about how EQCM and EQCM-D are used in battery development and help researchers take battery performance to the next level.
Read about how QSense EQCM-D analysis was used to explore the build-up, evolution, and mechanical properties of the solid electrolyte interphase (SEI).
Read about how QSense EQCM-D, Electrochemical Quartz Crystal microbalance with Dissipation monitoring, was used to analyse new battery electrode materials.
Calendering, a common compaction process for Li-ion batteries, will significantly impact the pore structure and thus also the wettability of the electrode.
