Throughout drug manufacturing, storage and administration stages, protein drugs may interact with many different types of surfaces such as glass, oils, plastic polymers, and metals in concentrators, tubings, containers or bags.
Studying protein interaction with the different surface materials and at different solution conditions, the material compatibility can be evaluated and conditions that minimize for example adsorption can be identified. It also enables tailoring of formulation additives, such as stabilizers and surfactants, as well as of the packaging used in the development and manufacturing of new protein therapeutics. Here we show examples where QSense® QCM-D analysis was used to do such assessments.
Quartz Crystal Microbalance with Dissipation monitoring, QCM-D, is a well-established surface sensitive technology which has been used for analysis of molecular interactions at surfaces and interfaces for more than two decades. The time resolved information on mass, thickness and viscoelastic properties can for example be used to analyze molecular adsorption and desorption, layer build-up, and to characterize layer properties.
Below we briefly present four studies where QSense technology was used to analyze and quantify the adsorption of therapeutic monoclonal antibodies and proteins to different materials and interfaces. In addition to the impact of the material, the effect of sample concentration and presence of surfactants on the surface uptake was also characterized and quantified.
Analysis of antibody adsorption to different surfaces and at different conditions
In this study,1 the adsorption behavior of two monoclonal antibodies, mAb1 and mAb2, with significant differences in hydrophobicity and self-oligomerization behavior were evaluated. The surface uptake of the two molecules was characterized as a function of surface material, presence/absence of surfactant, concentration and pH.

Influence of molecule: In the antibody study,1 it was concluded that the amount adsorbed varied with the antibody used. On all of the four surfaces analyzed, mAb2 showed a higher adsorption than mAb1 (Fig. 1A).
Influence of surface material: The results showed that the surface material significantly impacts the amount adsorbed. The highest amount of adsorption was found on Au, and the lowest was found on PS (Fig. 1A).
Influence of surfactant: The addition of surfactant significantly reduced the amount adsorbed for both antibodies. On the Au surface, both mAb1 and mAb2 adsorbed to a large extent even in the presence of surfactant (Fig. 1A).
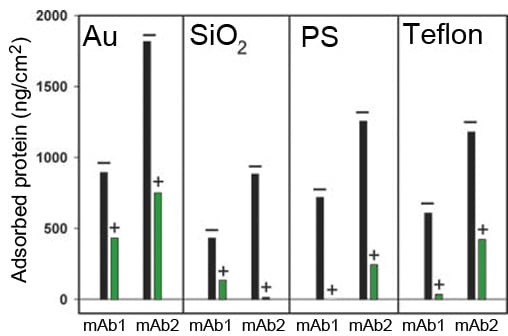
Figure 1A. Mass of irreversibly bound mAb1 and mAb2 (1 mg/ml). + and – represent the presence or absence of the surfactant PS-80.
Concentration dependence: There was a pronounced concentration dependence of the adsorption on PS, Teflon and Au surfaces. The concentration had less impact on the SiO2 surface (Fig. 1B).
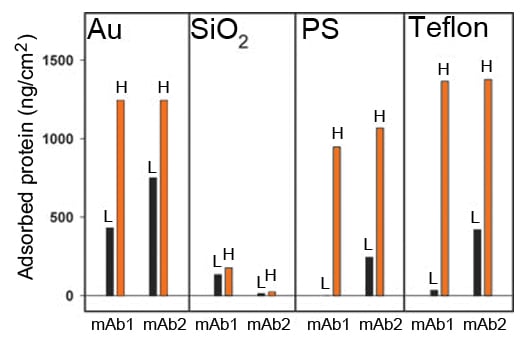
Figure 1B. Mass of irreversibly bound mAb1 and mAb2 as a function of concentration. L refers to 1 mg/ml and H refers to 50 mg/ml. All data was generated in the presence of PS-80. Control measurements provided an average value for adsorbed surfactant and protein mass has been adjusted accordingly.
Key observations: Combinations of antibody, surface material and excipient that essentially prevents adsorption were identified. The effect was most pronounced for mAb1 on PS.
Analysis of protein drug interaction with silicone oil with and without surfactant
In the second study,2 the interaction of a protein drug with a silicone oil/water interface was analyzed. A common way to store and administer therapeutic protein to patients is via prefilled glass-syringes. As silicone oil, which is used as a syringe lubricant, could induce protein loss, the protein-silicone oil interaction is relevant to evaluate. The adsorption of the protein to a silicone oil/water interface was characterized in the presence and absence of two surfactants.

Influence of surfactant: In the Abatacept study,2 it was concluded that the surfactant PS-80 reduced the amount of adsorption to the silicone oil/water interface whereas Poloxamer 188 did not alter the binding significantly (Fig. 2).
![]()
Figure 2. Changes in estimated mass adsorbed at the oil/water interface as a function of time upon injection of 10 mg/ml Abatacept without PS-80, with PS-80 and Poloxamer 188. This data was analyzed with permission from the authors.2
Analysis of protein adsorption to glass and plastics at low and high concentration
In the third study,3 protein adsorption to borosilicate and PVDF at low and high concentration was evaluated. The surface material and concentrations used are relevant to study in the context of protein production.

Influence of surface material: In the lyzosyme study,3 it was concluded that the surface uptake is higher on glass than on plastic for both concentrations, Fig 3.
Influence of protein concentration: The surface uptake was higher on both surfaces at the high concentration, Fig 3.
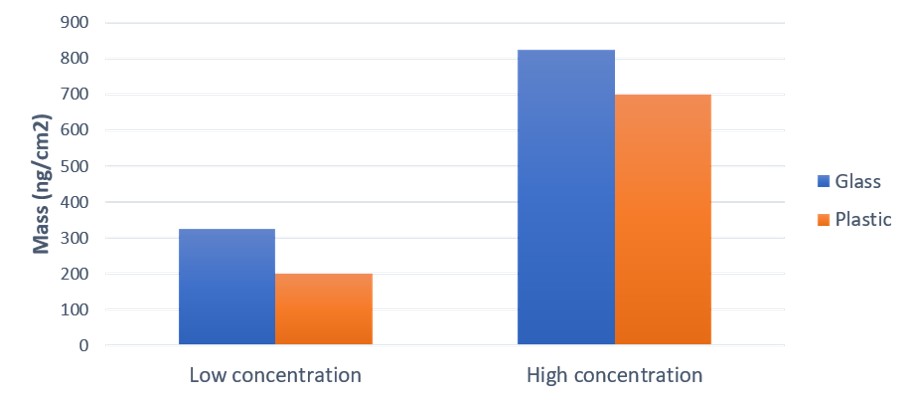
Figure 3. Protein adsorption, characterized as mass uptake, onto glass and plastics at two different concentrations, low (1 mg/ml) and high (40 mg/ml).
Analysis of monoclonal antibody adsorption to PDMS with and without surfactant at different concentrations
In the fourth study,4 the overall ambition was to develop a fundamental understanding of the aggregation propensities of mAb formulations, and to assess if surfactants of different type and concentration would impact the mAb adsorption and aggregation. The adsorption of two different monoclonal antibodies, mAb1 and mAb2, with different aggregation propensities, two pharmaceutically relevant surfactants, and a set of antibody-surfactant mixtures, was analyzed. The surface material, PDMS, served as a mimic of the silicone-oil coated surfaces encountered in pre-filled syringes. The surface mass uptake of the different samples was compared, and correlated, with additional and complementary analysis of the mAb surface activity and aggregation.

Antibodies: In the study,4 it was concluded that the surface mass uptake of mAb1 was higher than that of mAb2. It was also concluded that the adsorption essentially was irreversible, Fig 4A.
Surfactants: In the study, it was concluded that he mass uptake of the surfactants was lower than that of the mAbs. The surfactant adsorption was partially reversible, Fig 4B.
Antibody + surfactant mixtures: It was observed that the antibody and surfactants will co-adsorb, Figs 4B-C.
Key conclusions: In this work, the authors conclude that there is a direct correspondence between the adsorption of mAbs at oil-water interfaces and aggregation. They also concluded that added surfactants will competitively adsorb to the oil-water interface, and thereby lower the mAb aggregation, Fig 4.
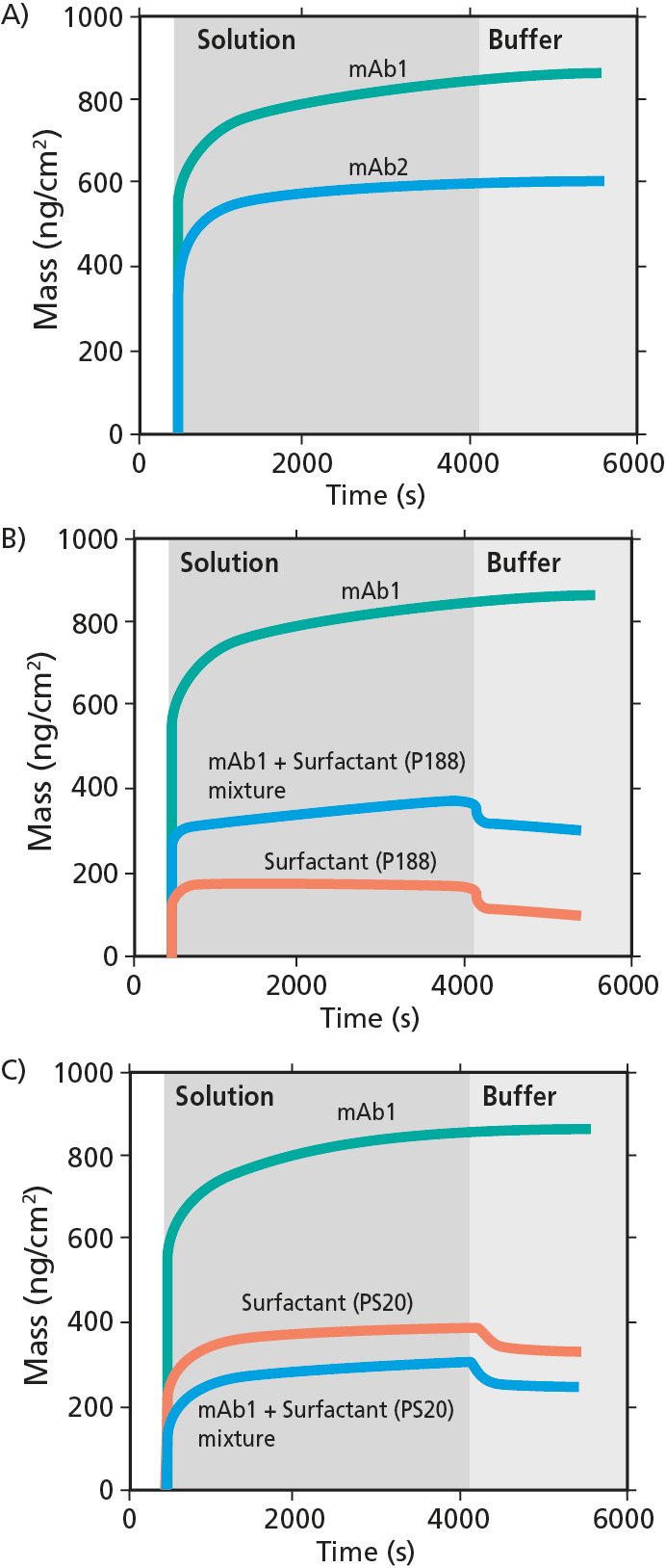
Figure 4. QSense analysis of mAb and surfactant adsorption onto PDMS. In A) mass uptake of mAb1 and mAb2. In B) mass uptake of mAb1 + surfactant (P188) mixture, and in C) mass uptake of mAb1 + surfactant (PS20) mixture.
QSense QCM-D technology has a long track record in analysis of molecular interactions at surfaces and interfaces. Here we have presented four very brief examples of what questions QCM-D analysis can help answer in the context of container and closure interactions.
Learn how QSense QCM-D reveals protein–surface interactions and adds interface-focused insight to biopharmaceutical formulation and stability work
Learn how QSense sensors enable application‑relevant biointerface interaction analysis and explore our sensor offering for different areas
Learn how QSense QCM D can be used to analyze swelling of thin films, including magnitude and dynamics.
Read about how molecule-surface interaction processes such as adsorption and desorption can be analyzed with QCM-D.
Learn best practices and step-by-step methods for accurate QCM-D coating thickness measurement on QSense sensors using QSense Omni.
Compared to QCM, QCM-D measures an additional parameter, and provides more information about the system under study.
Discover how QCM-D analysis reveals real-time etching dynamics, helping optimize cleaning processes and protect surfaces from unwanted damage.
Discover how QSense QCM-D helps tackle fouling challenges across industries
Discover how QCM-D enables real-time, label-free analysis of supported lipid membrane formation, structure, and dynamics for advanced research
