Wettability is the preference of a liquid to be in contact with a solid surrounded by another fluid (liquid or gas). Depending on the application, wettability can be wanted or not. Take for example a newly waxed car. The purpose of adding the wax is to prevent the water from spreading and to prevent corrosion of the car. In this case, the aim is clearly to reduce the wettability. Then, on the other hand, good wettability is needed when for example coating is applied on the surface (wax on top of the car).
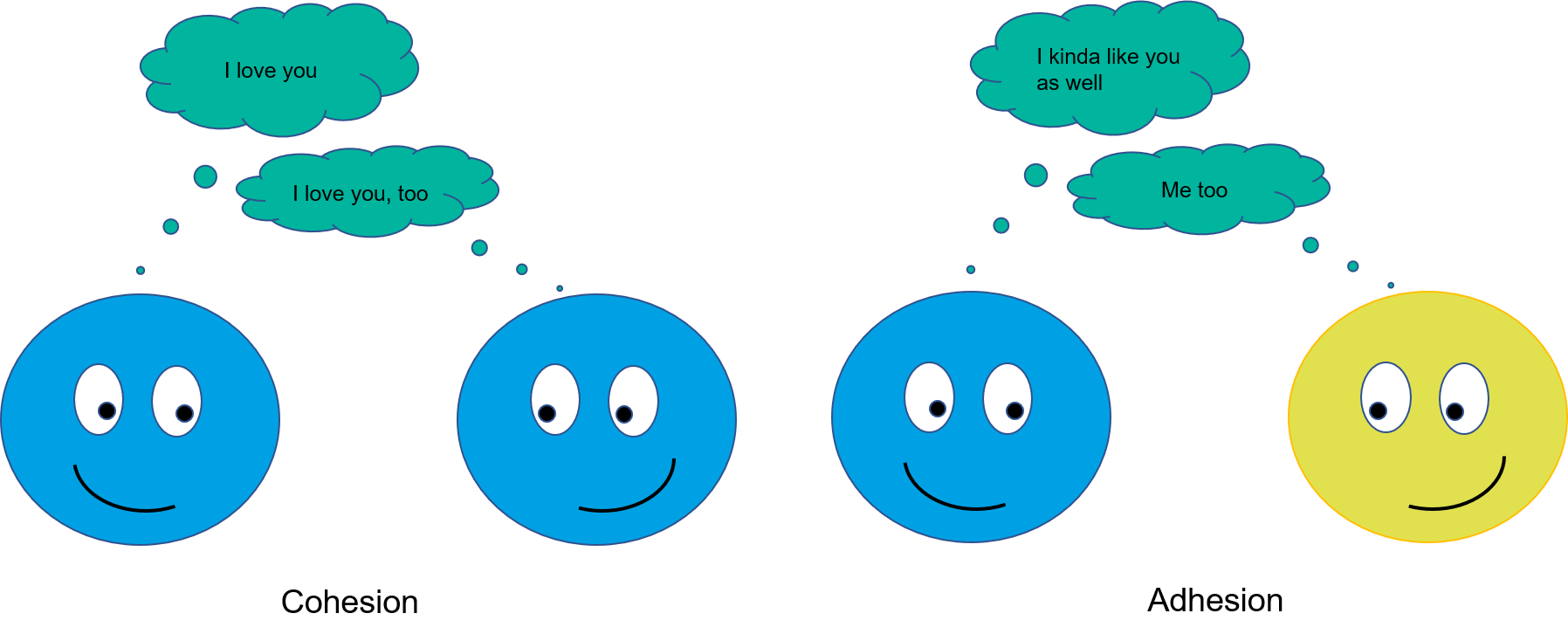
Adhesive and cohesive forces between the solid and the liquid determine the wettability. Cohesive forces affect in between the same type of molecules. In a liquid or solid, the molecules are pulled towards each other because of the cohesion between the molecules. Adhesion is the interaction between unlike molecules. The balance between these forces is what defines the degree of wettability. For example, if a drop of water on the solid substrate beads up, ethanol on the same substrate is likely to spread. This is because in the water there are strong cohesive forces called hydrogen bonds that pull the molecules towards each other. In ethanol, van der Waals forces are responsible for cohesive interactions and are much weaker than hydrogen bonds. This will cause ethanol to spread more easily on solid surfaces.
Solid also matters. Solids can be divided into high and low-energy solids. High energy solids such as glass, ceramics, and metals are held together by chemical bonds (covalent, ionic and metal) which are very strong. This causes high excess energy on the surface of the solid i.e. the name high energy solid. Most of the liquids will wet the high energy solid completely as that will lead to a decrease in interfacial energy. Low-energy solids, such as many of the polymers, especially Teflon are harder to wet.
The contact angle is a measure of wettability. A drop of liquid is placed on the solid and contact angle can be optically measured.  Typically, 90° contact angle is considered as a threshold value. When the contact angle is above 90° the wettability is bad, when it is below 90° the wettability is good. Complete wetting is achieved when the contact angle is zero, although in practice when contact angles are below 5° the surface is typically considered to be completely wetted. When water is used as a measuring liquid, the surface is called hydrophobic when the contact angle is above 90° and hydrophilic when it is below the same value.
Typically, 90° contact angle is considered as a threshold value. When the contact angle is above 90° the wettability is bad, when it is below 90° the wettability is good. Complete wetting is achieved when the contact angle is zero, although in practice when contact angles are below 5° the surface is typically considered to be completely wetted. When water is used as a measuring liquid, the surface is called hydrophobic when the contact angle is above 90° and hydrophilic when it is below the same value.
If you would like to read more about contact angles and how they can be measured, please download the white paper below.
Learn how to use contact angle measurements to evaluate surface cleanliness on different materials and link cleaning steps to reliable adhesion and coating quality.
Learn how wettability, surface free energy and surface roughness define paper and paperboard surface properties – and how to measure them for reliable print, coating and sealing performance.
Smart materials are materials that sense a change in their environment and respond in a useful, reversible way.
Discover how contact angle measurements help optimize plasma treatment for improved wettability and adhesion in industrial applications.
Discover why contact angle is essential for adhesion, coatings, and quality control. Learn how surface wettability impacts product performance.
Discover why PFAS-free coatings are needed, the challenges they present, and key strategies for developing high-performance alternatives.
At the heart of droplet formation are two key molecular forces: cohesion and adhesion.
Contact angle measurements provide a golden standard for evaluation of surface properties for quality control.
Contact angle is the angle a droplet forms in contact with a solid surface. Thermodynamically, it is a balance between cohesive and adhesive forces.
