
Unconventional oils, such as heavy oil, extra heavy oil, and bitumen, normally exist tightly on host solids such as rocks, sands and clay minerals. Successful liberation of unconventional oil from solids is essential for effective recovery. As shown in Figure 1, a typical oil liberation process using the aqueous phase as a flooding or processing medium from a sand grain can be divided into two sub-processes:
1) oil recession (displacement) on the sand surface in water;
2) and oil separation from the sand grain,
which are determined by the wettability of sand grains.
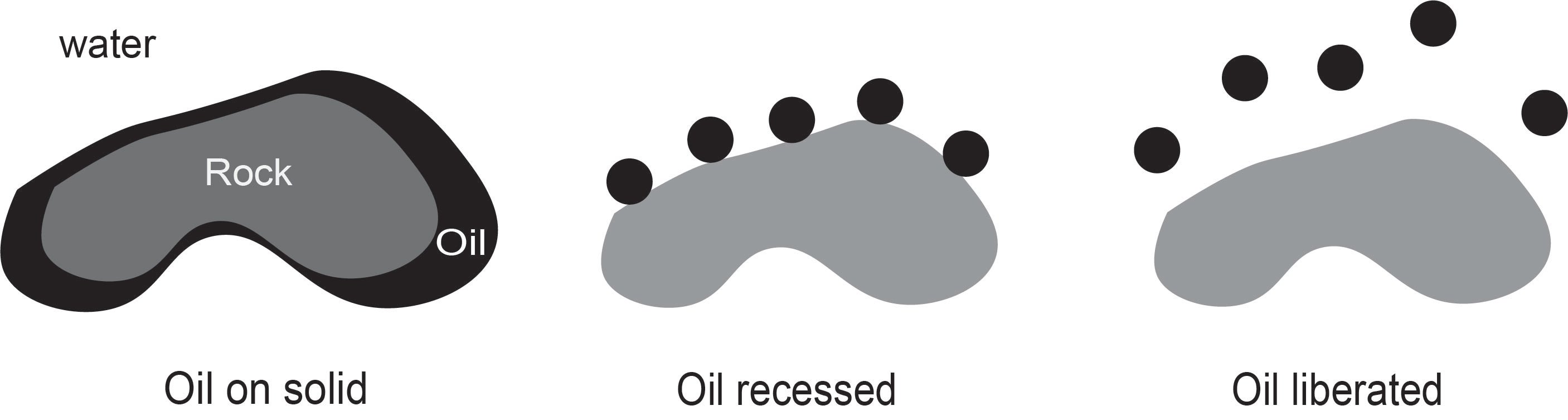
Figure 1 Oil liberation from a sand grain in two subprocesses; oil recession (displacement) and oil separation.
Wettability is defined as the relative ability of a fluid to spread on a solid surface in the presence of another immiscible fluid (air or liquid). Studying the wettability of a solid by liquid(s) is essential to understand the science of oil liberation from solid surfaces in heterogeneous systems involving oil-water-solid three-phase contact. In general, solid minerals in many unconventional oil reservoirs are intrinsically water-wet, which is favorable for oil liberation from the sand in water. The adsorption of asphaltenes and/or another surface-active organic components in the oil has a profound effect on altering the wettability of the solid surfaces to be oil-wet. Consequently, the efficiency of oil liberation from oil-wet solids is reduced dramatically, leading to poor recovery and product quality of the oil. Addressing this problem requires understanding and hence control of the wettability alteration of the reservoir solids.
Functional chemicals, salts, alkalis, and surfactants are commonly used to change the hydrophobic character of the solids to be hydrophilic in unconventional petroleum production. Based on the charge characteristics of polar groups, there are three main types of surfactants: cationic, anionic and nonionic. Recently, a new class of nanoparticle-based nanofluids was also found to be effective in altering the wettability of petroleum reservoir rocks1, 2. Temperature and pressure are known to play an important role in alteration of solid wettability.
An array of techniques, including Amott wettability test, United States Bureau of Mines (USBM) test and contact angle measurement have been effectively used to study the solid wettability. Among these techniques, contact angle measurements on smooth homogeneous surfaces are a direct and most widely accepted method to determine the wettability of solids measured by the contact angle. The contact angle at the oil-water-solid three-phase contact line is measured through the aqueous phase as shown in Figure 2 (a). Solids with the water contact angle close to zero can be considered to be completely water-wetted and the solids are classified as hydrophilic. On the other hand, solids are considered to be hydrophobic (oil-wet) if the water contact angle is larger than 90°. The solids of water contact angle around 90° are known to be bi-wettable, i.e., they partition readily at the oil-water interface as shown in Figure 2 (b).
Overall, solid wettability is one of the key factors in controlling the liberation of oil while the contact angle is the most universal measure of the wettability of solid surfaces and can be measured by optical tensiometer.
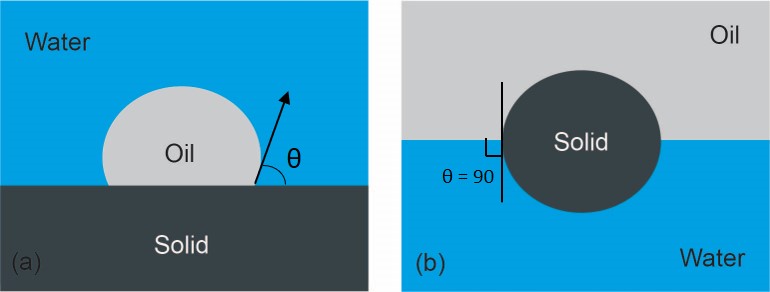
Figure 2 (a) Contact angle for solid-oil-water system with the contact angle defined as the angle measured through aqueous phase (b) Solid particle contact angle at oil-water interface.
To learn more about wettability and contact angles in the oil industry, please watch to webinar by Prof. Xu.
Nanoparticles alone or integrated with conventional enhanced recovery processes have shown promising performance in improving oil recovery.
Studies show the influence of EOR agents on the reservoir rock wettability. Studies are not considering the reservoir conditions i.e. high pressure.
There are three commonly used wettability measurement techniques for oil reservoir characterization; Contact angle, Amott-Harvey, and USBM.
Using so-called smart water flooding has increased interest in both sandstone and carbonate reservoirs due to its low cost and minimum impact on the environment.
Most commonly used methods to study reservoir wettability are Amott-Harvey, USBM, and sessile drop contact angle.
Carbonate reservoirs are characterized as intermediate to oil- wet. Altering the wettability of the carbonates has been proposed as one of the main mechanisms for enhanced oil recovery.
Different enhanced oil recovery methods are used to alter the wettability of the reservoir rock. To study the wettability alteration at the reservoir conditions, an instrument where the measurements can be done at high pressures and temperatures are needed.
Describes the instrument features needed for successful high-pressure interfacial tension measurements.
Dr. Zhenghu Xu is a professor at Southern University of Science and Technology in Shenzhen. His main research area is interfacial sciences as applied to natural resources processing and utilization.
