In our daily routines, we often reach for a familiar item to help us tackle the grime and grease that accumulate on our clothes, dishes, and skin: soap. This seemingly simple product goes by various names and comes in numerous forms, yet its purpose remains constant—to cleanse. Whether in bar, liquid, or powder form, soap plays a crucial role in maintaining cleanliness and hygiene in our lives. But have you ever stopped to ponder how this humble cleaner actually works its magic? In this blog post, we delve into the world of soap, exploring its composition, mechanisms, and the fascinating science behind its ability to rid surfaces of dirt and impurities.
Soap is a salt of fatty acids such as sodium stearate or sodium laureth sulfate. Fatty acid salts are molecules that have hydrophobic carbon tail and hydrophilic head groups. This will make molecules’ surface active meaning that they arrange at the air-water interfaces so that the hydrophobic tail is in the air and the hydrophilic head is in the water phase. When there is an excess amount of these molecules in a solution, the surface becomes saturated, and the molecules will arrange themselves into micelles. Micelle is like a reservoir of soap molecules, where hydrophobic tails are pointing inwards and hydrophilic head groups form a shell around it. The concentration at which micelle is formed is called critical micelle concentration and is an important parameter when cleaning formulations are developed.
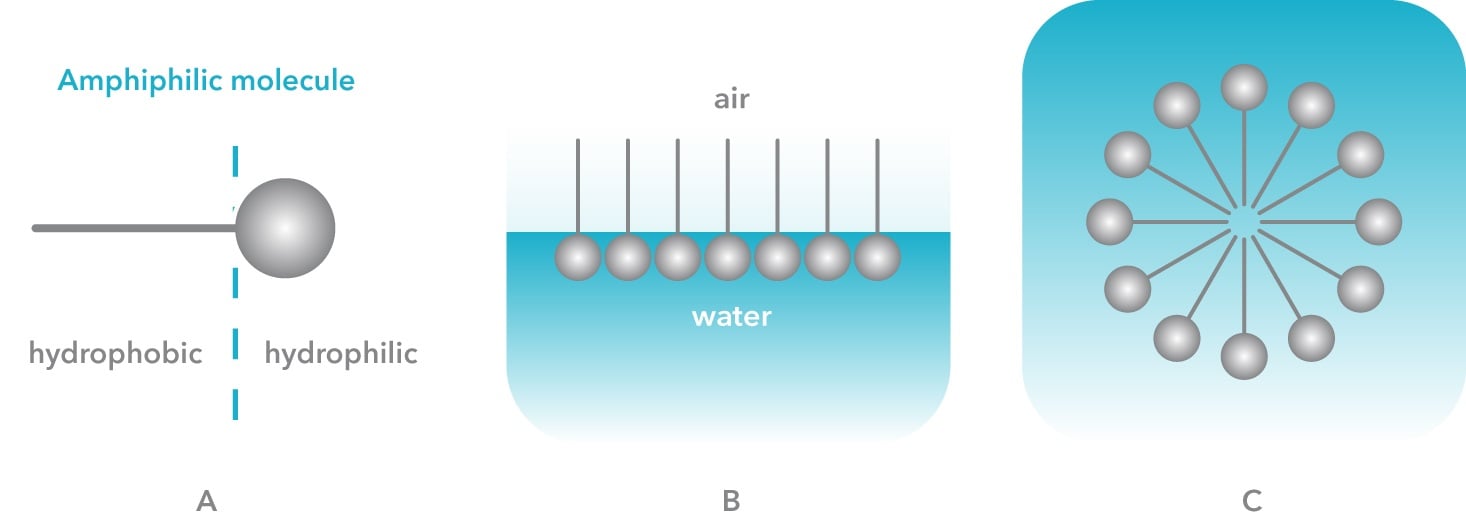
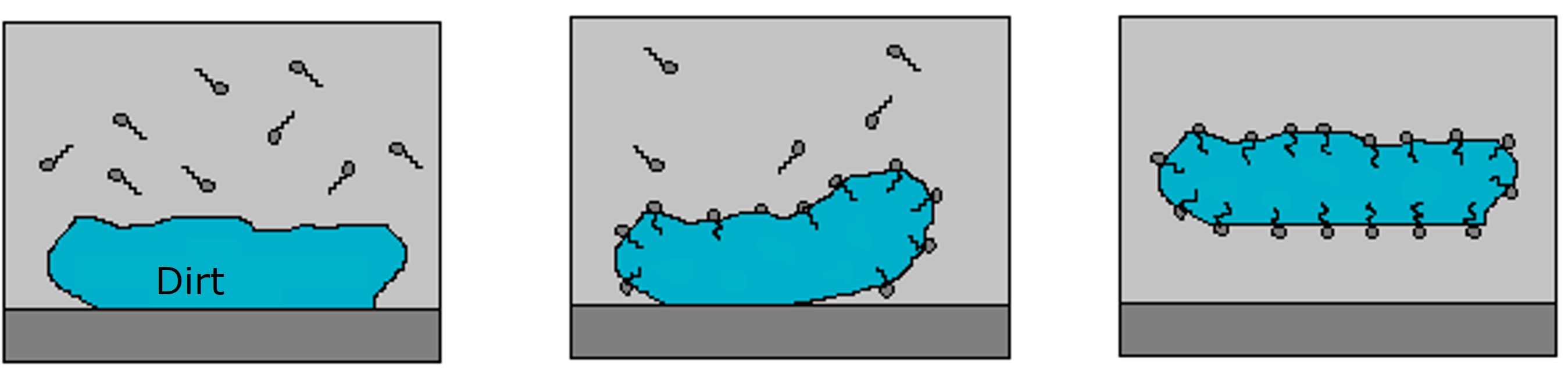
The hydrophilic and hydrophobic parts of the soap have a crucial role in the cleaning process. Typically, the dirt that is removed from our clothes and dishes is hydrophobic grease. When soap molecules in water come in contact with grease, they arrange themselves so that hydrophobic tails are inside the grease and hydrophilic heads point towards the water. While doing that, soap molecules decrease the adhesion force between the dirt and the substrate leading to less external work e.g. rubbing required to remove the dirt.
QSense QCM-D technology enables analysis of cleaning process dynamics, surface etching, and surface residual
QCM-D was used to compare the potency and mechanisms of action of two different detergents in disrupting lipid membranes
Cleaning product performance assessments can be time-consuming, but with the right approach, evaluation and ranking can take less than one hour.
Read about Prof. Jackman's experience using QCM-D to study surfactant-interaction with model membranes
Read about the different components in cleaning products and how they work on a molecular level.
Soil removal efficiency of a formulation is typically assessed by running a cleaning test followed by an analysis of the result. Here we show how these two steps can be run simultaneously.
The traditional way of assessing etching properties can be time-consuming. But there are faster ways. Here we present a method that will help you assess the etching effects in one single wash cycle and in less than an hour.
What is the optimal surfactant concentration to use in a cleaning formulation? Here we show how the cleaning efficiency as a function of detergent concentration can be assessed within an hour.
