
QSense QCM‑D measures swelling of thin films by tracking changes in resonance frequency (Δf) and dissipation (ΔD) of a quartz sensor as the film takes up or releases solvent. The water or solvent uptake is sensed as an apparent mass increase and a change in viscoelastic properties of the layer at the surface, recorded in real time.
The function and properties of many natural and man‑made materials depend on their ability to take up and release water or solvent. Examples include hydrogels in biomaterials and tissue engineering, thickeners and emulsifiers in food and cosmetics, hygroscopic films and coatings in the chemicals industry, and filtration and separation devices, all relying on controlled hydration and dehydration.
In research and product development, it is therefore highly relevant to study swelling processes of these materials, for example to understand mechanical stability, permeability, dissolution, fouling or cleaning behavior. Because swelling is an interfacial phenomenon that changes both the mass and mechanical properties of a film, it is well suited for study with QSense QCM‑D, a surface‑sensitive and label‑free technology which can be used to characterize and optimize water uptake and release processes in thin films.
The amount of solvent, for example water, in a thin film can be very high, sometimes >95%, depending on the molecules present and their conformation at the surface. As an illustrative example, consider elongated molecules that adsorb flat on the surface compared to the same molecules adsorbing in a more upright fashion, Fig. 1.:
The hydration and dehydration of molecular layers, and the transitions between more collapsed and more hydrated states, can be characterized with QCM‑D. In such measurements, water uptake and swelling are sensed as an apparent mass increase at the surface (Δf↓) together with a change in dissipation that reflects the softer, more hydrated character of the swollen film.
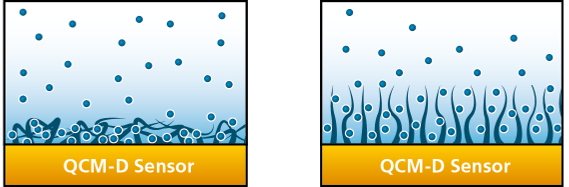
Figure 1 (conceptual). Molecules adsorbed flat onto a surface (left) couple less solvent than when adsorbed in an upright or more open conformation (right). The latter conformation allows more water to enter the film, contributing to swelling.
Starch is an important ingredient in, for example, the paper industry and in the design of edible films. In both areas, the effect of ambient humidity on material strength and quality is a central question:
In the example below,1 a thin film of native potato starch (NPS) on a QSense QCM‑D sensor is exposed to air at different humidity levels while the change in film thickness is monitored, Fig. 2. The thickness is obtained by modelling the measured Δf and ΔD response with an appropriate viscoelastic model.
This measurement illustrates how humidity induces swelling of the film and affects its thickness, and how the material responds when conditions are reversed. The results provide insight into how the material behaves under different humidity conditions, which is directly relevant for product performance in use. 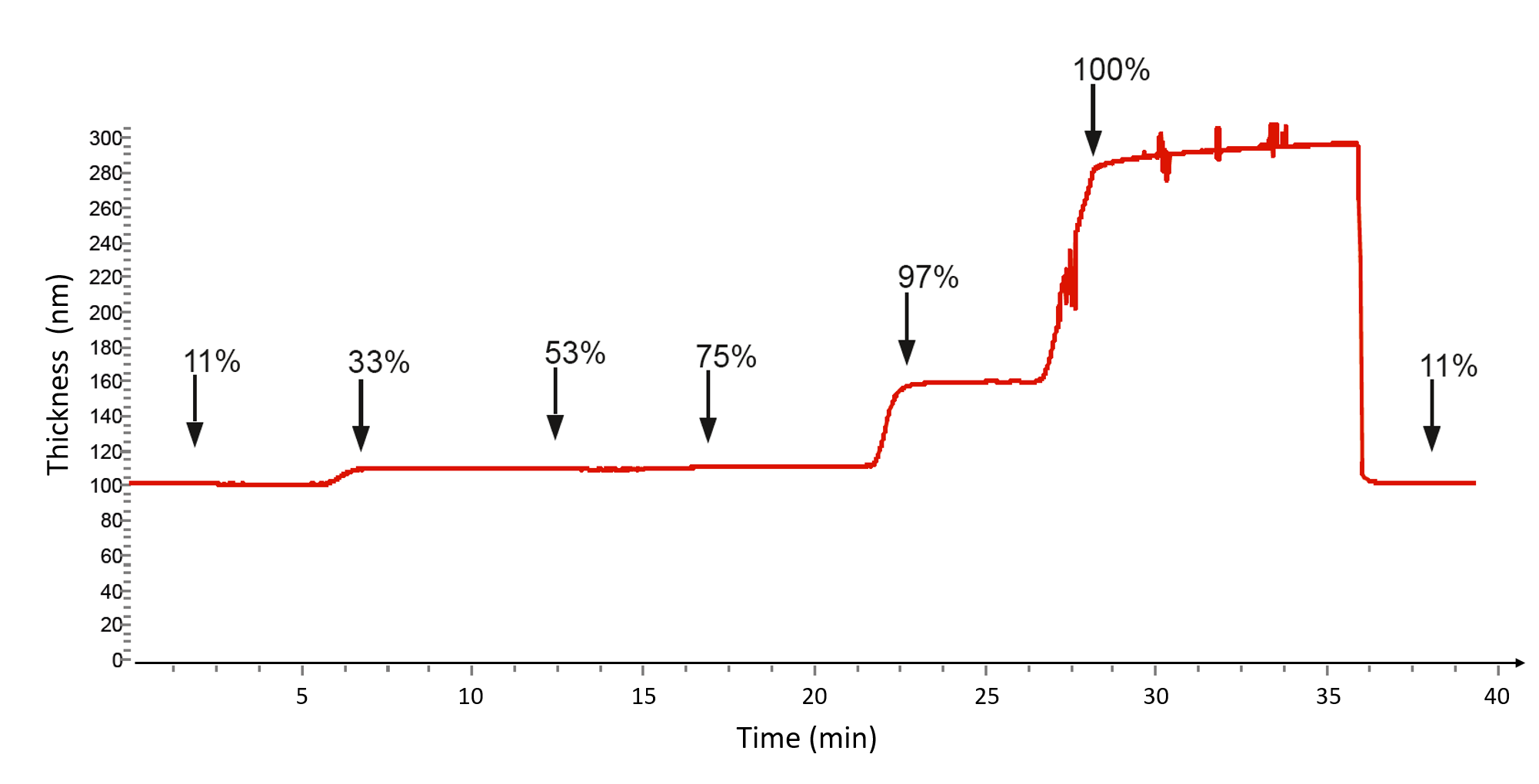 Figure 2. Analyzing vapor uptake and thin film swelling using QCM-D.
Figure 2. Analyzing vapor uptake and thin film swelling using QCM-D.
When a film swells due to solvent uptake, QCM‑D typically records:
When the film collapses or dehydrates (for example, when humidity is lowered again):
By following these signals at different overtones, QCM‑D provides information on:
A generic protocol to study swelling of thin films with QCM‑D can look like this:
1. Prepare the thin film on the sensorMeasurements of vapor uptake, solvent uptake and thin film swelling are relevant in many areas where hygroscopic or responsive materials are used. Examples include:
In all these contexts, understanding how and how much a material swells, and whether that process is reversible, is important for designing robust and high‑performing products and processes.
QSense QCM‑D provides a straightforward and sensitive way to monitor swelling and collapse of thin films. By following the mass (Δf) and dissipation (ΔD) in real time, you can quantify how much a film swells, how fast it responds, how reversible the swelling is, and how the mechanical properties of the layer change as it hydrates or dehydrates. This information is valuable across a wide range of research and development contexts where interfacial hydration, film stability and solvent response play a critical role in product and process performance.
Download the overview to read more about what phenomena you can study and what information you can obtain with QSense QCM-D.
FAQ
Q: How does QCM‑D measure swelling of thin films?
A: QCM‑D measures swelling by tracking changes in resonance frequency (Δf) and dissipation (ΔD) as the film takes up or releases water or solvent. Swelling typically appears as a decrease in frequency (apparent mass increase) and an increase in dissipation as the film becomes softer and more hydrated.
Q: What information can I obtain about swelling with QCM‑D?
A: From a swelling experiment you can obtain the magnitude of swelling (relative or absolute thickness change if modelled), the dynamics of swelling and collapse, the reversibility and how the mechanical properties of the film change as it hydrates or dehydrates.
Q: Which types of films can I study with QCM‑D in this context?
A: You can use QCM‑D to study swelling and deswelling of thin films and layers such as hydrogels, starch and other polysaccharides, synthetic polymers, coatings, soil/deposit layers, responsive films and many other nm–µm interfacial layers, as long as they are rigidly attached to the sensor surface.
Q: In which applications is swelling analysis with QCM‑D particularly useful?
A: Swelling analysis with QCM‑D is particularly useful in biomaterials and biointerfaces (hydrogels, polymer brushes, bio‑inspired coatings), in coatings and specialty chemicals, in cleaning where soil layers swell before removal, and in water and environmental technologies where membranes and adsorbent layers interact with water and humidity.
Editor’s note: This post was originally published in 2018 and has been updated for comprehensiveness
Learn how QSense QCM-D reveals protein–surface interactions and adds interface-focused insight to biopharmaceutical formulation and stability work
Learn how QSense sensors enable application‑relevant biointerface interaction analysis and explore our sensor offering for different areas
Read about how molecule-surface interaction processes such as adsorption and desorption can be analyzed with QCM-D.
Learn best practices and step-by-step methods for accurate QCM-D coating thickness measurement on QSense sensors using QSense Omni.
Compared to QCM, QCM-D measures an additional parameter, and provides more information about the system under study.
Discover how QCM-D analysis reveals real-time etching dynamics, helping optimize cleaning processes and protect surfaces from unwanted damage.
Discover how QSense QCM-D helps tackle fouling challenges across industries
Discover how QCM-D enables real-time, label-free analysis of supported lipid membrane formation, structure, and dynamics for advanced research
