In recent years, micellar water has taken the beauty and skincare industry by storm, touted as a miracle product for gentle yet effective cleansing. What makes micellar water so special, and why has it become a staple in skincare routines worldwide? In this blog post, we will delve into the science behind micellar water and unravel its cleansing magic.
Micellar water is a clear, water-like liquid that contain one or several different types of surfactants. 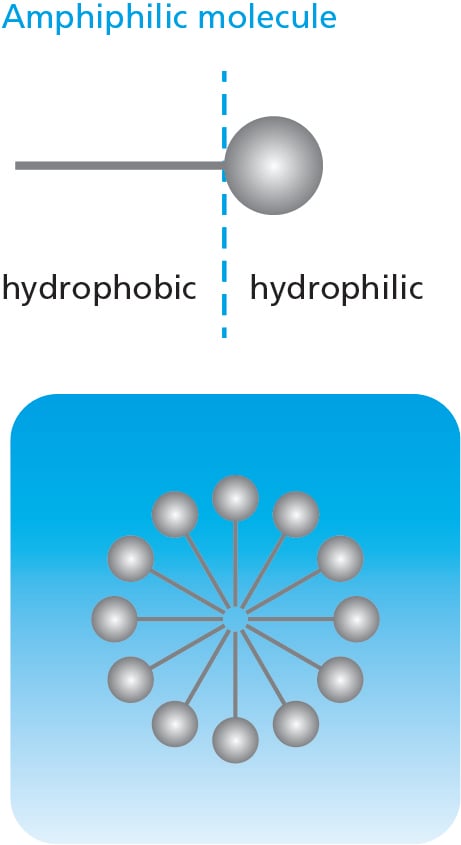 Surfactants are molecules which have a hydrophilic (water-attracting) "head" and a lipophilic (oil-attracting) "tail." When there is an excess amount of surfactants in a solution, they form micelles. As the name implies, micelle have a crucial role in the cleaning process. Micellar water is used as a makeup remover and general facial cleanser. It is often free of harsh chemicals, making it a gentle option for daily cleansing and suitable for most skin types.
Surfactants are molecules which have a hydrophilic (water-attracting) "head" and a lipophilic (oil-attracting) "tail." When there is an excess amount of surfactants in a solution, they form micelles. As the name implies, micelle have a crucial role in the cleaning process. Micellar water is used as a makeup remover and general facial cleanser. It is often free of harsh chemicals, making it a gentle option for daily cleansing and suitable for most skin types.
When micellar water is applied to the cotton pad, the micelle breaks and hydrophilic parts are attracted to the water (towards the pad) whereas lipophilic parts are pointing outwards. As the pad is swiped across the skin’s surface, the lipophilic tails seek out oil, dirt, and other impurities on the skin. The oil and dirt particles are surrounded and encapsulated by the surfactants. Now, the hydrophilic head is again pointing outwards making the impurities suspended within the liquid and efficiently removed from the skin.
The science behind micellar water reveals why it has become a beloved skincare product. Its micellar structures effectively remove impurities, makeup, and excess oil from the skin's surface without the need for harsh scrubbing, making it suitable for a wide range of skin types. Its versatility and hydrating properties have made it a must-have in skincare routines worldwide. So, the next time you reach for that bottle of micellar water, remember the fascinating science that makes it such a gentle yet powerful cleanser for your skin.
Discover what biosurfactants are, how they work in pollution cleanup, why they’re the sustainable choice, and how their effectiveness is measured.
Discover how QCM-D enables real-time cleaning analysis and reveals surfactant performance to optimize cleaning product development
Emulsifiers are surfactants that stabilize oil-water interfaces.
Surfactants are utilized in numerous products from cleaning formulations to paints and pesticides.
Discover how QSense QCM-D technology reveals real-time cleaning insights. Join our webinar to enhance your cleaning strategies and efficiency.
QSense QCM-D technology enables analysis of cleaning process dynamics, surface etching, and surface residual
QCM-D was used to compare the potency and mechanisms of action of two different detergents in disrupting lipid membranes
Explore a case example of surfactant adsorption with QSense Omni, showcasing its performance and enhanced data quality.
QCM-D is utilized to understand the compatibility and to identify solution and excipient conditions that minimize the adsorption of therapeutic proteins
