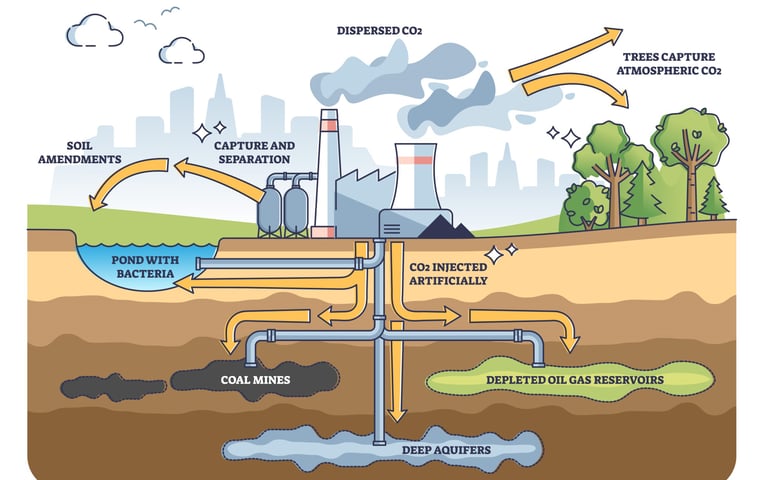
Carbon capture and storage (CCS) or CO2 sequestration has become an important research topic as global warming causes concern around the globe. The natural way of storing CO2 is of course done by nature with forests and oceans. The natural way is however not enough to compensate the amount of CO2 the usage of fossil fuels produces. Few different approaches have been proposed such as depleted oil and gas reservoirs and deep saline acquires. With all the approaches successful CO2 storage is mainly related to fluid-fluid and fluid-rock interfacial interactions. As CO2 is also often used for enhanced oil recovery, the same research efforts serve both purposes as the interactions are between the same fluids and solids, brine, CO2/oil, and the rock.
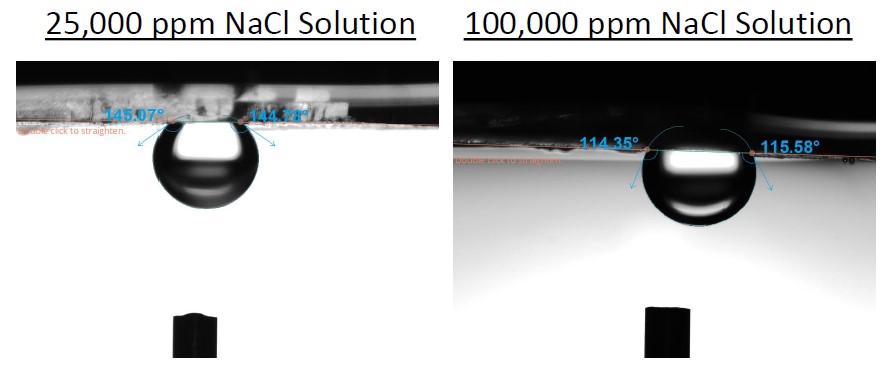
For long-term trapping of the CO2, it is crucial to understand the contribution of different functional trapping mechanisms which prevent the buoyant CO2 from migrating back to the surface. It has been shown by various researchers that the water-wet surface chemistry is beneficial for the CO2 trapping process [1]. Water wetness is easily studied through contact angle measurements. Contact angle measurements offer a fast method to study the effects of salinity and different rock surface chemistries on wettability. The contact angle measurements are done by immersing the rock or rock mimic material, typically quartz and calcite on brine and placing a drop of oil on the sample surface. When measurements are done in this way, the higher contact angles mean more water-wet substrate. From the two figures below, it could then be concluded that lower salinity leads to better CO2 sealing capacity.
Contact angle measurements can also be performed under various pressures and temperatures. For this, the optical tensiometer is equipped with a high-pressure measurement cell. Such an instrument allows one to study the interactions between brine, CO2, and rock directly. It has been shown that the storage capacity of the reservoir is directly dependent on the contact angle between the water and CO2 through equation
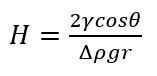
, where h is the CO2 column height, γ is the interfacial tension between the water and CO2, θ is the water contact angle on a rock surrounded by CO2, Δρ is the density difference between water and CO2, g is the acceleration due to gravity, and r is the radius of the pores [2]. The higher column height means better CO2 sealing capacity which can be achieved by lowering the contact angle of water on a rock surrounded by CO2. Using a high-pressure cell thus allows you to study the effect of several variables such as pressure, temperature, salinity, and rock surface chemistry with a relatively short measurement time.
[1] K. Chaudhary, M. B. Cardenas, W. W. Wolfe, J. A. Maisano, R. A. Ketcham, and P. C. Bennett, "Pore-scale trapping of supercritical CO2 and the role of grain wettability and shape," Geophysical Research Letter, vol. 40, no. 15, pp. 3878-3882, 213.
[2] M. Ali, S. Al-Anssari, M. Arif, A. Barifcani, M. Sarmadivaleh, L. Stalker, M. Lebedev, and S. Iglauer, "Organic acid concentration threshold for aging of carbonate minerals: Implications for CO2 trapping /storage," Journal of Colloid and Surface Science, vol. 534, pp. 88-94, 2019.
Discover what carbon capture and storage (CCS) is, how it works, and why it’s vital for cutting CO₂ emissions and meeting climate targets.
Interfacial tension and wettability are important parameters determining the success of the CO2 storage site.
