Emulsions and foams are dispersed systems commonly encountered in various industries and everyday life. Emulsions consist of two immiscible liquids, such as oil and water, while foams are made up of gas bubbles dispersed in a liquid. Both can be found in numerous applications, from food products to industrial processes.
Understanding and controlling the stability of emulsions and foams are vital for optimizing product quality and process efficiency across various sectors. Whether enhancing the texture of a food product or ensuring the smooth operation of an industrial process, managing these dispersed systems is key to achieving desired outcomes.
Emulsions appear in many forms, from food items like mayonnaise and salad dressings to personal care products such as day creams and lotions. In the industrial sector, emulsions are crucial in the production of paints and coatings, ensuring smooth application and consistent performance. However, emulsions can also form as unwanted byproducts in processes like crude oil extraction, where their presence can complicate operations.
The stability of emulsions is essential when they are intentionally created, as it directly affects the quality and functionality of the final product. Conversely, in situations where emulsions are undesirable, destabilizing agents are necessary to break them down and restore efficiency to the process.
Foams, comprising gas bubbles dispersed in a liquid or solid matrix, are equally widespread. In the food industry, foams contribute to the texture and structure of products like whipped cream, meringues, and certain breads. In cosmetics, foams are used in products like shaving cream and mousses. Industrial applications include firefighting foams.
Like emulsions, the stability of foams can be either beneficial or problematic. Stable foams are critical for the desired performance of products like shaving creams or culinary foams. On the other hand, unwanted foams can interfere with manufacturing processes, requiring the use of antifoaming agents to break them down.
Emulsions are formed by agitating two immiscible liquids, such as oil and water, in the presence of an emulsifier. Emulsifiers can be proteins, phospholipids, or even nanoparticles. There are two primary types of emulsions: oil-in-water (O/W), where oil is the dispersed phase and water is the continuous phase, and water-in-oil (W/O), where the roles are reversed. The type of emulsifier used determines the type of emulsion formed.
The hydrophilic-lipophilic balance (HLB) of a surfactant measures how hydrophilic (water-attracting) or lipophilic (oil-attracting) it is. Hydrophilic surfactants are water-soluble and act as emulsifying agents for O/W emulsions. In contrast, lipophilic (or hydrophobic) surfactants are oil-soluble and act as emulsifying agents for W/O emulsions. This balance is crucial in designing and stabilizing emulsions for various applications.
Emulsions can be destabilized by four primary mechanisms: creaming/sedimentation, flocculation, coalescence, and Ostwald ripening.
1. Creaming/Sedimentation:
Mechanism: This occurs due to the density difference between the dispersed and continuous phases.
Impact of creaming: In oil-in-water (O/W) emulsions, the lighter oil droplets rise to the surface.
Impact of sedimentation: In water-in-oil (W/O) emulsions, the denser water droplets settle at the bottom.
2. Flocculation:
Mechanism: Emulsion droplets aggregate, forming larger clusters without merging.
Impact: While the droplets remain distinct, their aggregation can lead to instability and eventual separation if further destabilization occurs.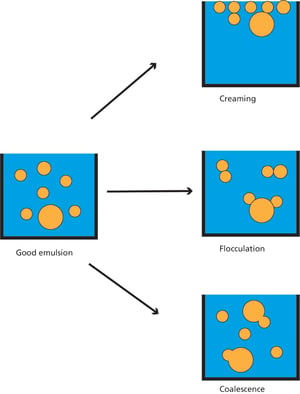
3. Coalescence:
Mechanism: Smaller droplets merge to form larger droplets. This happens when droplets collide, and the interfacial film between them ruptures, leading to phase separation over time.
Impact: Continuous merging of droplets results in larger droplets and eventual separation of the emulsion into distinct phases.
4. Ostwald Ripening:
Mechanism: Smaller droplets dissolve in the continuous phase and then redeposit onto larger droplets. This process occurs because it leads to a more thermodynamically stable state, where the system minimizes its total interfacial energy.
Impact: Over time, this leads to an increase in the average droplet size and can contribute to the breakdown of the emulsion.
Understanding these mechanisms is crucial for controlling emulsion stability, whether the goal is to maintain the emulsion in products like food and cosmetics or to break down unwanted emulsions in industrial processes. Interfacial properties between the liquid-liquid or air-liquid interfaces play a role in the stabilization process. Interfacial rheology studies have been utilized to better understand the phenomena at the interfaces.
If you are interested in learning how emulsion stability can be predicted, please watch our webinar from the link below.
This blog was originally published on the 24th of November, 2020. It has since been updated for comprehensiveness.
The same measurement modes used in bulk rheology are also meaningful in interfacial rheology.
Pickering emulsions utilize solid particles to stabilize the interface between the two immiscible liquids
International Congress on Interfacial Rheology was held in Athens from the 29th of July to the 4th of August 2023.
Foam stability refers to the ability of a foam to maintain its structure and resist collapse over time.
In this blog post, the most common interfacial shear rheology methods are reviewed.
Interfacial rheology studies the response of the interfacial layer to the external stimuli at air-liquid or liquid-liquid interfaces.
Interfacial shear rheology at the gas-liquid or liquid-liquid interface is relevant in a wide range of applications where foams and emulsions are used.
Interfacial rheology is a special branch of rheology that involves studying the unique two-dimensional systems formed at interfaces.
