When surface-active molecules are present in the solution, they tend to adsorb in the gas-liquid or liquid-liquid interface. Surface active agents have hydrophobic and hydrophilic parts. When at the interface, they orient so that the hydrophilic part is in a water-based solution and the hydrophobic part in the air or oil phase.
Surface active agents can be surfactant molecules, polymers, proteins, or even particles. When those molecules or particles adsorb at the interface, the properties of the interface change.
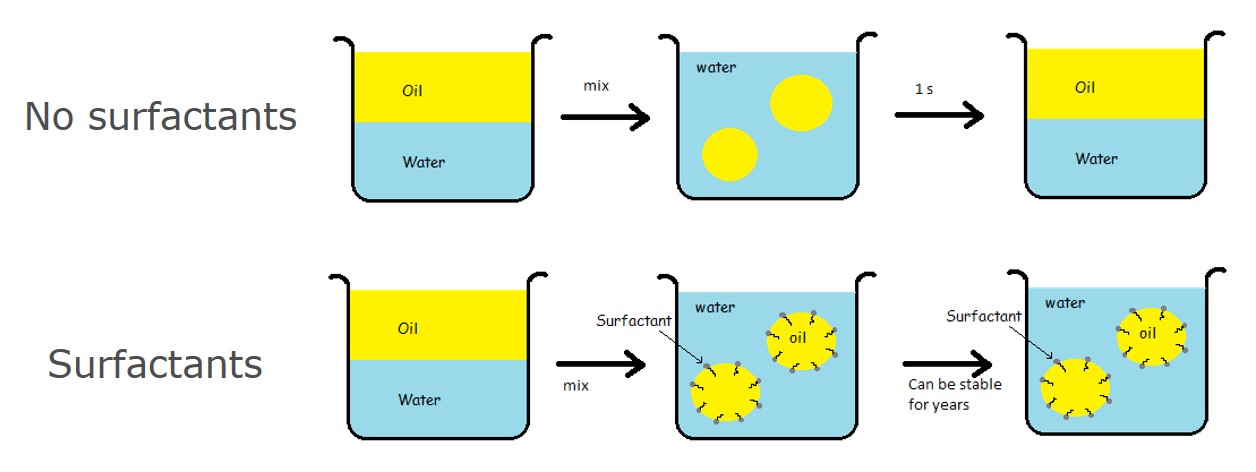
A simple example to think about is having oil and water in a beaker. As they don't mix, and oil is lighter than water, the oil will sit on top of the water layer. If you mix the oil and water together, you can for a short time see separate oil drops in water but as you let it sit on the table, the phases will soon separate again. The oil-water interface is not stable enough to keep the oil drops separate, and as the oil drops collide with each other, they will immediately merge together.
Adding surface-active agents will change the situation. When oil and water are mixed together, the surface-active molecules will adsorb at the interface of the oil drops. Now, when oil drops collide with each other they don't immediately merge together as the interfacial layer will work as a barrier. Depending on the surface-active agents, such a system can be stable for a very long time.
The system described above is called emulsion. If you are working with emulsions, you have probably been struggling with emulsion stability. Depending on the application, the emulsion stability can be wanted or unwanted. In both cases, the way to understand the stability is through interfacial rheology measurements.
Interfacial rheology studies the response of the adsorbed interfacial layer to the deformation caused by external stimuli. There are several ways to deform the interface. Whichever method is used, the important thing is to follow how the deformation changes the properties of the interface. The response depends on the layer composition. By selecting the appropriate layer composition one can form a more stable emulsion and thus improve the quality of the product. Or find a way to break an unwanted emulsion.
To learn more about emulsion stability and how it can be studied with interfacial rheology, please watch the webinar through the link below.
Editorial note: This blog post was first published in May 2017 and has been updated for improved accuracy
Emulsions are dispersed systems of two immiscible liquids such as oil and water. Interfacial rheology measurements predict emulsion and foam stability.
The same measurement modes used in bulk rheology are also meaningful in interfacial rheology.
Pickering emulsions utilize solid particles to stabilize the interface between the two immiscible liquids
International Congress on Interfacial Rheology was held in Athens from the 29th of July to the 4th of August 2023.
Foam stability refers to the ability of a foam to maintain its structure and resist collapse over time.
In this blog post, the most common interfacial shear rheology methods are reviewed.
Interfacial rheology studies the response of the interfacial layer to the external stimuli at air-liquid or liquid-liquid interfaces.
Interfacial shear rheology at the gas-liquid or liquid-liquid interface is relevant in a wide range of applications where foams and emulsions are used.
