
When water hits the surface, it can behave in several different ways. It can either spread on the surface completely, roll off the surface, or stay there as a droplet. Depending on the shape of the drop, more precisely, the angle that the water drop takes as it sits on the surface, the surface is termed either hydrophilic or hydrophobic. The words come from Greek. Hydro- stands for water, and -philic comes from the word philos, which means to love. A hydrophilic surface can thus be considered as water-loving which makes a water droplet spread on it. Hydrophilic surface properties are needed in many different applications ranging from biomedical devices to marine engineering.
The hydrophilicity of the surface is determined by the functional groups that are available to interact with the water drop. Hydrophilic functional groups include hydroxyl-, carbonyl-, carboxyl-, amino, sulfhydryl-, and phosphate groups. Multiple materials such as glass or other ceramics are inherently hydrophilic, but it is possible to render the surface hydrophilic with different types of surface treatments. For example, plasma treatment is often used to make polymer surfaces hydrophilic, at least temporarily.
Hydrophilicity is often wanted when coatings are applied or before the bonding process. This is because the coating formulation spreads better on hydrophilic surfaces filling possible cavities as well. The proper wetting of the surface (i.e. spreading of the formulation) is required to achieve good adhesion between the coating and the substrate.
Hydrophilic surface properties are also often preferred in biomedical applications as they can prevent protein adsorption as well as improve the lubricity of the urinary catheters.
Anti-fouling surfaces are very important not only in biological applications but also for example in marine engineering as they can prevent the build-up of micro-organisms to the bottom of a ship. A thin layer of water forms a barrier that prevents or delays the attachment of micro-organisms.
Anti-fogging surfaces are another example where hydrophilic surface properties are sometimes preferred. Anti-fogging surfaces prevent fogging in applications where good visibility is important such as a windshield or swimming goggles.
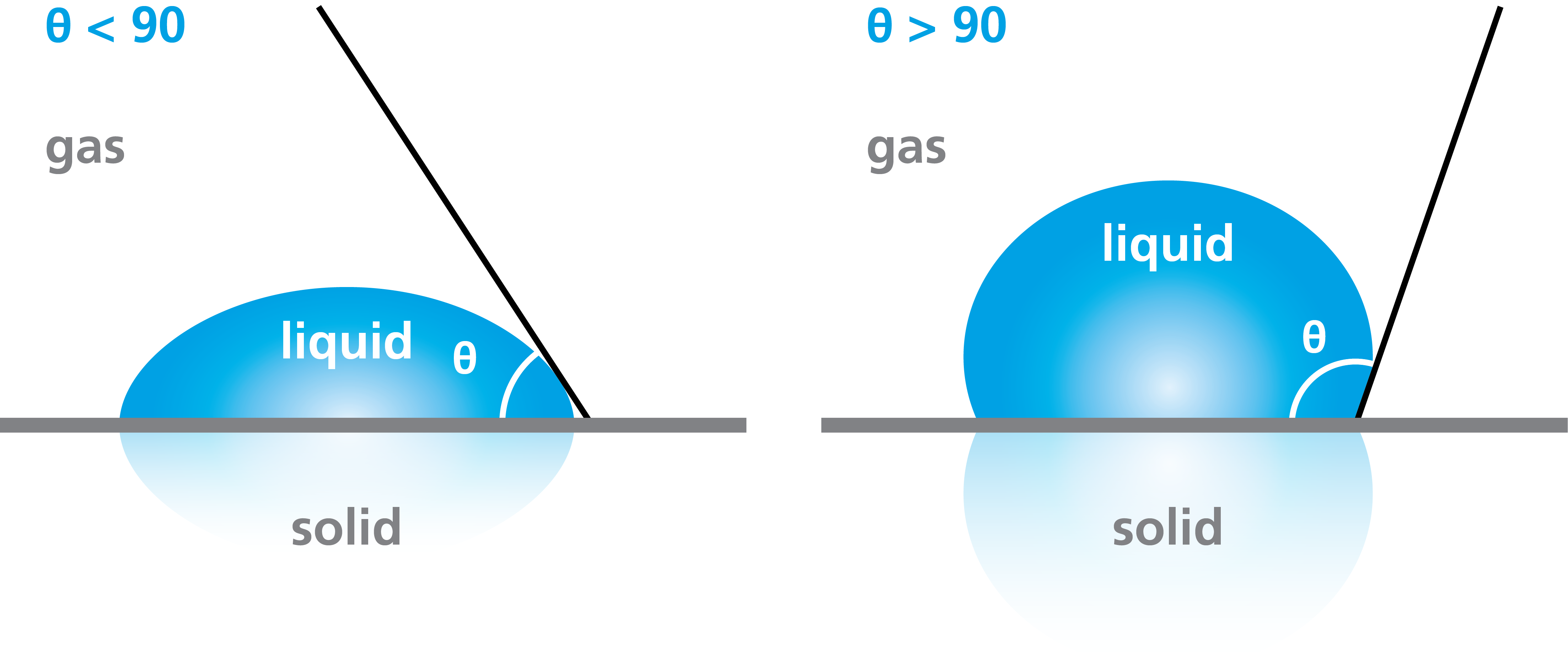
As already discussed, the surface is termed hydrophilic when the water drop spreads on it. The degree of surface hydrophilicity (or -phobicity) can be determined by the contact angle measurement. The surface is said to be hydrophilic if the contact angle is below 90 degrees. If the contact angle is lower than 5 degrees the surface is completely wetted and called superhydrophilic.
If you want to read more about contact angle and its measurement methods, please download the white paper through the link below.
Learn about the effect of surface roughness and wettability on biocompatibility of biomaterials and medical devices.
This blog post describes the importance of fiber diameter on the contact angle measurements of fibers with Wilhelmy method
Standard contact angle measurement considers the surface's chemical properties. The influence of surface roughness is added by utilizing the Wenzel equation.
Wettability is crucial in biomedical applications as it affects protein adsorption, cell adhesion, blood coagulation, and bacterial colonization.
Liquids’ ability to wet a solid surface has widespread importance in many everyday products and industrial processes.
Membrane wettability is a key property to ensure success of membrane distillation process
Hydrophobic surface properties are needed in contact with the food product while hydrophilicity is needed when printing on food packaging.
Wettability is pivotal in pharmaceutical dosage form manufacturing as well as in drug efficacy.
Understanding the wettability of membranes is essential for optimizing these processes and achieving desired separation outcomes.
