
The knowledge of surface tension, contact angle, and viscoelastic properties of emulsions, solutions, and foams have great importance in food science. Outputs of surface characterization are critically important for research and industrial-scale applications.
Interfacial tension can be defined as the force interaction between molecules at the interface of two fluids. If the force at the air-liquid interface is measured, it is called surface tension. Since interfacial and surface tensions measure the force per interfacial length, they have milli Newton per meter (mN/m) as the SI unit.  Interfacial tension gives us comprehensive information for the preparation of different food solutions. It plays a major role in the evolution of the interfacial area between the dispersed and continuous phases in a sheared emulsion. It strongly affects the two major phenomena that impact drop size; drop breakup, and droplet-droplet coalescence, which directly affects the stability of food products. For example, if any salad dressing, mayonnaise, high protein beverage, or ice cream are prepared, it is extremely important to know interfacial tension/surface tension.
Interfacial tension gives us comprehensive information for the preparation of different food solutions. It plays a major role in the evolution of the interfacial area between the dispersed and continuous phases in a sheared emulsion. It strongly affects the two major phenomena that impact drop size; drop breakup, and droplet-droplet coalescence, which directly affects the stability of food products. For example, if any salad dressing, mayonnaise, high protein beverage, or ice cream are prepared, it is extremely important to know interfacial tension/surface tension.
The contact angle is another important parameter in a diverse range of food applications. The contact angle is one of the common ways to measure the wettability of a surface or material. Wetting refers to the study of how a liquid behaves on the substrate, whether it spreads out or the ability of liquids to form boundary surfaces with the solid. The contact angle is an angle that a liquid creates with a solid surface or capillary walls of a porous material when both materials come in contact together. This angle is determined by both properties of the solid and the liquid and the interaction and repulsion forces between the liquid and solid and by the three-phase interface properties (gas, liquid and solid).
Those interactions are described by cohesion and adhesion forces which are intermolecular forces. The balance between the cohesive forces of similar molecules such as between the liquid molecules (i.e., hydrogen bonds and Van der Waals forces) and the adhesive forces between dissimilar molecules such as between the liquid and solid molecules (i.e., mechanical, and electrostatic forces) will determine the contact angle created in the solid and liquid interface.
Contact angle measurements are critical in food packaging applications. The tendency of food to interact with its packaging is a significant factor that can affect quality, appearance, shelf life, and microbiological safety. Adherence of food residues to the packaging may decrease product acceptability, enhance oxidation, and off-flavors, increase waste, and result in lower overall product quality. In addition to this, a product’s higher affinity to the wrapping material could result in an increased risk of migration of some of the package compounds, or in enhanced adsorption of off-flavors.
The tendency of food to interact with its packaging is a significant factor that can affect quality, appearance, shelf life, and microbiological safety. Adherence of food residues to the packaging may decrease product acceptability, enhance oxidation, and off-flavors, increase waste, and result in lower overall product quality. In addition to this, a product’s higher affinity to the wrapping material could result in an increased risk of migration of some of the package compounds, or in enhanced adsorption of off-flavors.
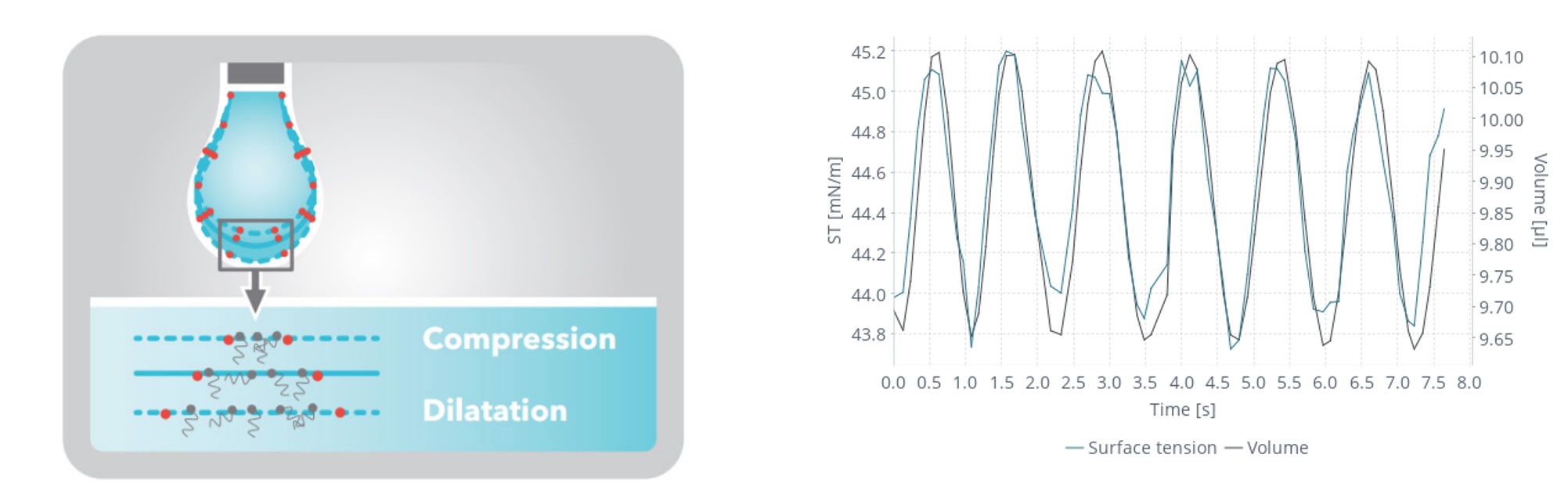
The application of foams and emulsions constitutes a wide area of use in food science. The performance and shelf life of these items are heavily influenced by their stability. Surfactants, proteins, and polymers, which are amphiphilic molecules, are important components in these emulsions and foams. To reduce their free energy, these molecules will preferentially adsorb to the air-liquid or liquid-liquid interface in a certain orientation. These materials will flow across each other during storage and consumption, changing the size and shape of their interfaces on a regular basis. Interfacial rheology is the study of how these molecules respond to external deformations and stimuli. A droplet containing surface-active chemicals will expand and contract during dynamic processes. The number of molecules adsorbed on the air-liquid or liquid-liquid interface fluctuates as the surface area of the droplet changes. These amphiphilic molecules' hydrophilic heads will gravitate toward water or polar liquids, while their hydrophobic tails will orient toward air or oil phases. The rate at which this occurs is a good indicator of a foam's or emulsion's stability. If surfactant molecules reach the interface fast, for example, there are fewer opportunities for a droplet to mix with another neighboring droplet, resulting in increased stability.
Foams can generally be thought of as pockets of gas trapped by a liquid or solid. For liquids, the stability of the interface is a key driver for the overall stability of the foam, and this is influenced by the viscosity, surface tension, and drainage of the liquid or entrapping phase. In food science, foams are present in many forms. Meringue, which is a type of dessert or candy, is often associated with Swiss, French, Polish, and Italian cuisines, whipped cream, mousse, and visible large macro forms in cappuccinos or lattes are the most common foam structures.
To hear more about these measurements in food science, please watch the short webinar through the link below.
We introduce the four different techniques that have been identified to study the wettability of the milk powder.
Read about the complexity of the microbiome, and resent research on how to make a better probiotic.
