
In short; surface tension is the property of the liquid in contact with gas phase (usually air). Interfacial tension, on the other hand, is the property between any two substances. It could be liquid-liquid, liquid-solid or solid-air.
Surface and interfacial tension are usually presented by the symbol σ and it is measured by force per unit length. Its SI unit is millinewton per meter (mN/m) which is equivalent to often used cgs unit, dynes per centimeter (dynes/cm).
To understand the origin of surface and interfacial tension, we need to understand two additional terms; cohesion and adhesion.
Cohesion is the attraction between molecules that are like each other. Cohesive forces are intermolecular forces which causes liquid to resist separation. For example, in water, there are strong cohesive forces, hydrogen bonds, that causes the rain to come down as a water drop rather than a mist.
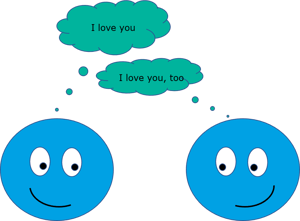
The cohesive forces between the molecules are responsible for surface tension. Or, the imbalance of these forces as there are less neighboring molecules on the surface than in the bulk of the liquid.
Cohesive forces on solids are so strong that, solids don't stick to materials they come in contact. Liquids, like water, on the other hand, has also adhesive forces, which get them to interact with other liquids and solids.
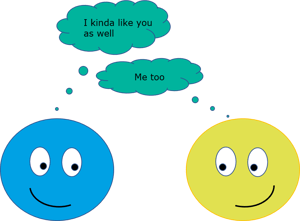 Adhesion is the interaction between unlike molecules. Adhesion force is caused by forces acting between two substrates, such as mechanical and electrostatic forces. When two immiscible liquids are brought into contact, the adhesion forces play a role. Adhesion forces are also important when liquid is brought in contact with solid.
Adhesion is the interaction between unlike molecules. Adhesion force is caused by forces acting between two substrates, such as mechanical and electrostatic forces. When two immiscible liquids are brought into contact, the adhesion forces play a role. Adhesion forces are also important when liquid is brought in contact with solid.
Both surface and interfacial tension are important in many industrial applications. Surface tension, for example, is measured when critical micelle concentration needs to be determined. Interfacial tension on the other hand is important parameter in emulsion stability.
If you are interested in reading more about surface and interfacial tension and their measurement techniques, please download the white paper below.
A wetting agent is a surface-active molecule used to reduce the surface tension of water.
The term surfactant comes from the word surface active agent. At the interface, they align themselves so that the hydrophobic part is in the air and the hydrophilic part is in water. This will cause a decrease in surface or interfacial tensions.
Surface tension plays an important role in Li-ion battery slurry optimization.
Surface tension plays an important role in the electroplating solution.
When measuring contact angles or making surface tension measurements with a pendant drop, selecting the correct tip or needle for your liquid is crucial.
The surface tension of water is about 72 mN/m at room temperature which is one of the highest surface tension for liquid.
Surface tension is a quantitative measure that can be correlated with a solution’s ability to remove dirt.
Surface tension and wettability are important physical properties that play a significant role in the effectiveness of agrochemicals.
Explains three different methods to measure surface tension.
