Have you ever overfilled your glass of water so that the water level is higher than the glass and still the water remains in the glass? This seemingly gravity-defying observation indicates there is a force that wants to keep the liquid together. This force is called the surface tension.
Imagine the interface between a liquid and a gas. Most of the molecules in both phases are in the bulk but some of the molecules are at the interface facing the other phase. The molecules in the bulk interact similarly with all the molecules surrounding them – there is a similar pull to all directions resulting in zero net force.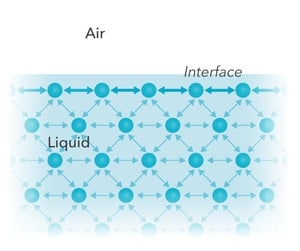
On the contrary, the molecules at the interface experience a stronger pull towards the bulk of their own phase. The net force, which effectively aims to keep the liquid together, is called surface tension.
Mathematically, surface tension is the force exhibited by the intermolecular forces divided by the length of the contact line between the phases. Therefore, the unit of surface tension is N/m. The forces are generally so small that the unit of mN/m has established itself as the standard unit being used. Water, for example, has a relatively high surface tension of 72 mN/m in room temperature due to its strong hydrogen bonds.
Many of the measurement methods for surface tension are also based on directly measuring the force exhibited by the surface tension per length of a measurement probe.
To learn more about what is surface tension and how it can be measured, please download the white paper below.
A wetting agent is a surface-active molecule used to reduce the surface tension of water.
The term surfactant comes from the word surface active agent. At the interface, they align themselves so that the hydrophobic part is in the air and the hydrophilic part is in water. This will cause a decrease in surface or interfacial tensions.
Surface tension plays an important role in Li-ion battery slurry optimization.
Surface tension plays an important role in the electroplating solution.
When measuring contact angles or making surface tension measurements with a pendant drop, selecting the correct tip or needle for your liquid is crucial.
The surface tension of water is about 72 mN/m at room temperature which is one of the highest surface tension for liquid.
Surface tension is a quantitative measure that can be correlated with a solution’s ability to remove dirt.
Surface tension and wettability are important physical properties that play a significant role in the effectiveness of agrochemicals.
Explains three different methods to measure surface tension.
Jyrki Korpela is the Global Product Manager for Attension and KSV NIMA. He has a background in biomaterials from Aalto University and is constantly looking for ways to make the life of customers easier and their results better. He’s excited about working at the frontiers of science and progress
