Lithium-ion batteries are the main energy storage technology for mobile devices such as smartphones and laptops. A recent increase in demand for plug-in-hybrids and electrical cars has initiated the discussion of whether the lithium-ion battery technology will ever be good enough for mass-market full electrification.
Lithium-ion battery composes of porous positive and negative electrodes which are filled with electrolyte solution and separated by a separator. The positive electrode, cathode, is typically constructed from Lithium Cobalt Oxide or Lithium Manganese Oxide. The negative anode is traditionally made of graphite or other carbon-based material. The electrolyte is commonly lithium salt in an organic solvent. Polymer-based electrolytes are often used. The electrolyte allows only Li-ions to move between the anode and the cathode. A separator is a physical barrier between the anode and the cathode. While cathode and anode determine the performance of the battery, electrolyte and separator are responsible for the safety of the battery.
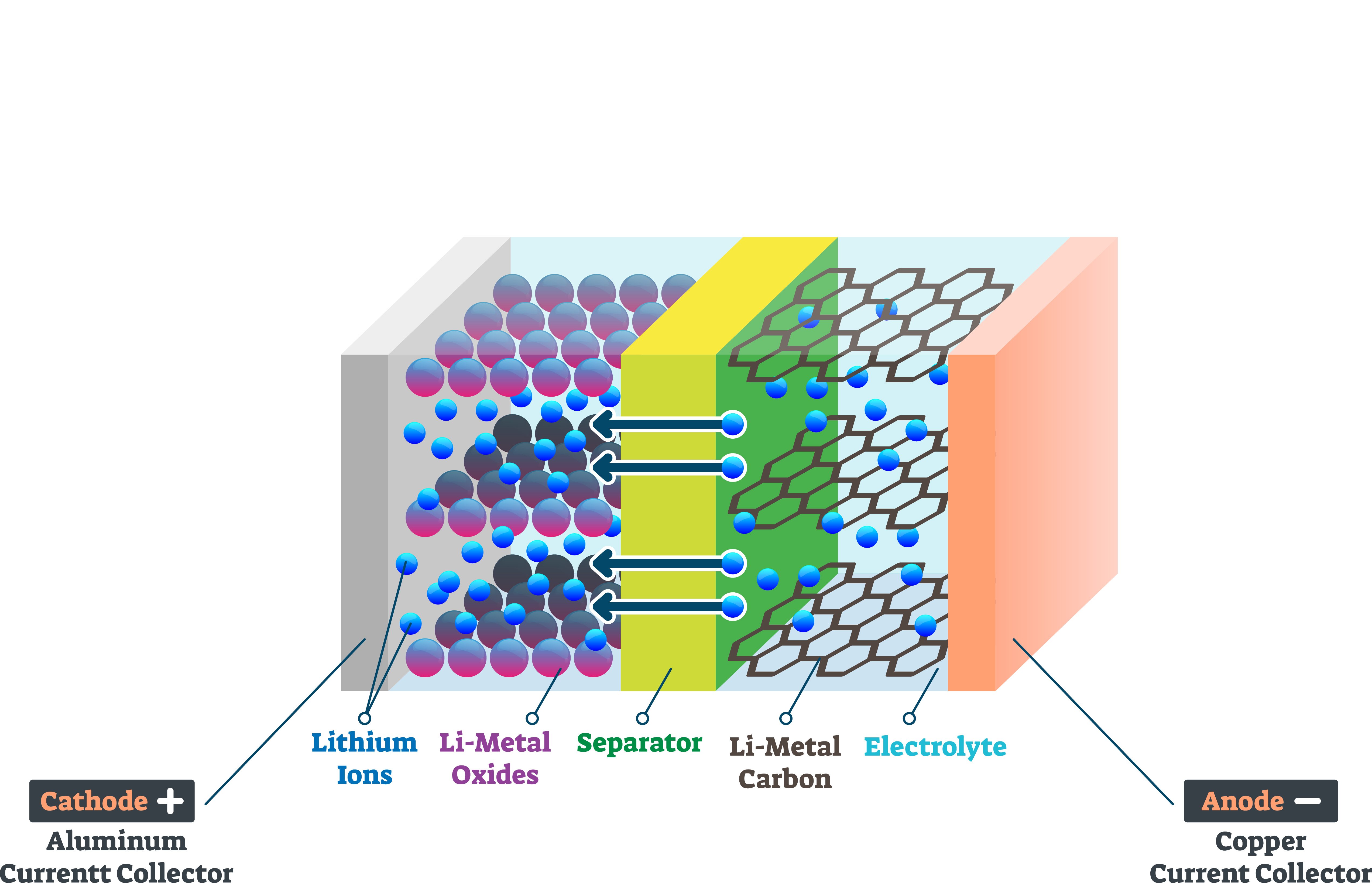
The wettability of different parts of Li-ion batteries has become one of the key issues both in terms of manufacturing as well as for the performance and safety of the batteries.
Wettability of the electrode material with the electrolyte solution is one of the challenges in the development of high-performance lithium-ion batteries.
Transfer from small-sized batteries into large-scale applications for electric vehicles poses significant challenges to battery manufacturing. One of the key steps in manufacturing is the addition of the electrolyte solution into a porous electrode by a precision pump. In this step, the electrolyte should permeate and fill the pores of the electrode. This process is called a wetting process and can take several days at elevated temperatures because of the poor wettability of the electrode, long diffusion distances, and hindered diffusion as gases are trapped within pores. The long process will increase the manufacturing time and at the same time the costs of manufacturing.
In addition, insufficient electrolyte wetting of porous electrodes leads to irregular reactions in the electrodes and unstable formation of the solid-electrolyte interface film. This can deteriorate the cell performance and cause poor cycle life. In addition, incomplete wetting enables the dendrite formation of lithium metal, which causes severe safety issues. Unwetted active material will also lead to underutilization of electrode capacity and increase electrode resistance.
A separator is a key component of the battery as it is placed between positive and negative electrodes. It prevents the battery short circuit by blocking the physical contact between the two electrodes but at the same time allows the flow of lithium-ion. A separator has been considered as an inactive component of the battery, but its properties are of utmost importance for the performance and safety of the batteries.
A separator is a porous membrane between electrodes of opposite polarity. A variety of different separator materials have been used over the years but today's commercial separators are commonly made of polyolefins, such as polyethylene or polypropylene.
The wettability by electrolyte is a critical characteristic of lithium-ion battery separator as electrolyte adsorption is essential for ionic transport. Polymeric separator materials are inherently hydrophobic with insufficient wettability to conventional organic electrolytes. Different approaches have been considered to increase the wettability of the separator material. These include different types of coatings using for example electrospinning or atomic layer deposition (ALD), and fabrication of composite separators.
Both force and optical tensiometers can be used to study the wettability of Li-ion batteries. To understand how wettability can be studied, please download the white paper through the link below.
Learn about the effect of surface roughness and wettability on biocompatibility of biomaterials and medical devices.
This blog post describes the importance of fiber diameter on the contact angle measurements of fibers with Wilhelmy method
Standard contact angle measurement considers the surface's chemical properties. The influence of surface roughness is added by utilizing the Wenzel equation.
Wettability is crucial in biomedical applications as it affects protein adsorption, cell adhesion, blood coagulation, and bacterial colonization.
Liquids’ ability to wet a solid surface has widespread importance in many everyday products and industrial processes.
Membrane wettability is a key property to ensure success of membrane distillation process
Hydrophobic surface properties are needed in contact with the food product while hydrophilicity is needed when printing on food packaging.
Wettability is pivotal in pharmaceutical dosage form manufacturing as well as in drug efficacy.
Understanding the wettability of membranes is essential for optimizing these processes and achieving desired separation outcomes.
