
Understanding the wettability behavior of powders is important in many application areas such as inks and paints as well as in food industry and in the pharmaceutical industry. Wettability is the preference of a liquid to be in contact with a solid surrounded by another fluid. Wetting is the first step of dispersion and dissolution of a powder and thus its complete understanding is important for instance when assessing bioavailability of a drug or suitability of a pigment to a paint.
Wettability of a solid surface is commonly measured with an optical tensiometer by utilizing the sessile drop method. A water droplet is placed on the surface and the formed contact angle is measured from the three-phase boundary where liquid, gas and solid intersect. Wettability of powders can also be measured with optical tensiometer but can be challenging due to the porous and loose structure of powders which makes the droplet often spread and sink into the powder instantly. The most common way to measure powder wettability is to use the Washburn method with force tensiometers. However, the best method to characterize powder wettability depends on the powder properties and application purposes.
Washburn method is the most applied and attractive method to determine the wettability of powders as it is fast, automated and cost-efficient. The method is based on capillary rise wetting where a powder packed into a specific container is considered as a bundle of parallel capillaries of constant radius. 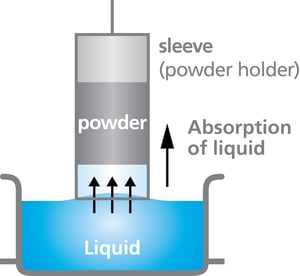 The container with a permeable bottom is brought in contact with the liquid and the mass of liquid absorbed into the powder is measured against time. The resulting curve provides information about absorption quantity and kinetics which depend on the contact angle of the powder and the liquid properties.
The container with a permeable bottom is brought in contact with the liquid and the mass of liquid absorbed into the powder is measured against time. The resulting curve provides information about absorption quantity and kinetics which depend on the contact angle of the powder and the liquid properties.
Contact angle, θ, can be defined from the Washburn equation
cosθ=η/(Cρ2 γl) *m2/t,
where C is material constant, ρ is density of the liquid, η is viscosity of the liquid, γl is surface tension of the liquid and m2/t is the slope of the absorption curve. The material constant is unknown prior to the measurement but can be defined with a completely wetting liquid, like heptane that gives contact angle of zero. The material constant depends on the packing of the powder and the particle size and thus it is important to use the same packing technique for each sample. After obtaining the material constant, contact angle can be determined with the liquid of interest. The Washburn method is not suitable for hydrophobic powders, as the powder needs to be wetted for the liquid to rise into the powder bed.
Direct sessile drop measurements with optical tensiometer are fast to perform and require only a small amount of measurement liquid but can be challenging with powders. The droplet often penetrates the powder instantly. Thus, a high-speed camera is often needed to capture the initial contact angle before absorption.
If the powder is compressible, it can be compressed into a tablet format by applying pressure, after which the droplet can be placed on top of it. However, applying pressure and compressing the powder to a compact form might alter the surface properties of the powder particles and left the tablet surface rough. Thus, the compressed tablet might not reflect the actual surface properties of the powder. On the other hand, a compressed tablet might represent the end product better for certain applications. Direct sessile drop method can also be used to measure contact angle from loose powders. The powder can be simply spread on a surface with or without an adhesive. This way the powder is left intact and represents the real surface properties of the powder. Direct sessile drop measurements are most convenient for hydrophobic powders.
If you would like to read more about powder wettability measurements, please download the overview below.
Learn how to use contact angle measurements to evaluate surface cleanliness on different materials and link cleaning steps to reliable adhesion and coating quality.
Learn how wettability, surface free energy and surface roughness define paper and paperboard surface properties – and how to measure them for reliable print, coating and sealing performance.
Smart materials are materials that sense a change in their environment and respond in a useful, reversible way.
Discover how contact angle measurements help optimize plasma treatment for improved wettability and adhesion in industrial applications.
Discover why contact angle is essential for adhesion, coatings, and quality control. Learn how surface wettability impacts product performance.
Discover why PFAS-free coatings are needed, the challenges they present, and key strategies for developing high-performance alternatives.
At the heart of droplet formation are two key molecular forces: cohesion and adhesion.
Contact angle measurements provide a golden standard for evaluation of surface properties for quality control.
Contact angle is the angle a droplet forms in contact with a solid surface. Thermodynamically, it is a balance between cohesive and adhesive forces.
Henni works as a product specialist at Biolin Scientific. She has a master degree on biosystems and biomaterials engineering from Aalto University. She is an expert on contact angle and surface tension measurements.
