
Surfactants are key components in many products and processes where their surface-active properties are needed, and in such applications, the surfactant-surface interaction dynamic could be critical. Here we show how the surfactant interaction with surfaces can be analyzed in a time-resolved manner at the nanoscale.
In several products and processes, such as in detergents and cleaning agents, in pharmaceutical formulations, in oil recovery, CMP, as well as in mining, the surfactant-surface interaction dynamic is critical to the application. It is therefore relevant to understand these processes at the nanoscale. In this study, QSense QCM-D, which is a surface-sensitive real-time technology, was used to characterize the surfactant adsorption. Two different surfactants were analyzed, Triton-X and ßOG. The focus of the QCM-D measurements was to analyze:
The results, Figure 1, show that both Triton X-100 and ßOG adsorb to the surface. However, the time to saturation differs between the two, and Triton X-100 reaches saturation faster that ßOG even though the concentration was lower. The results also show that ßOG forms a thicker layer than the Triton X-100 and that more ßOG than Triton X-100 remains at the surface after rinse.
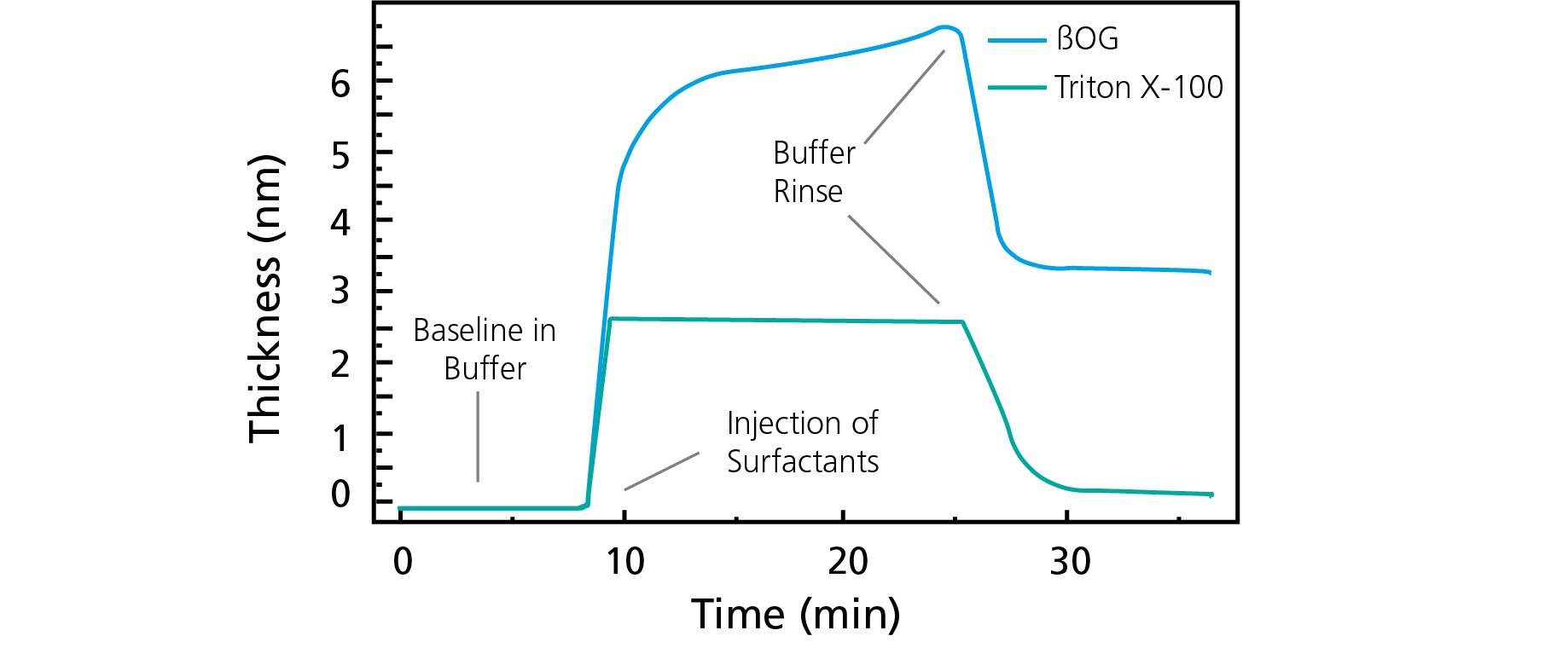
Figure 1. Time-resolved thickness change as the surfactants, Triton X-100 and ßOG, adsorb to the sensor surface.
In several products and processes, the surfactant-surface interaction dynamic is important and therefore relevant to understand at the nanoscale. QCM-D analysis provides information on surfactant-surface interaction processes under a variety of different substrate- and experimental conditions. Via this quantification of the surfactant-surface interaction, conclusions can be drawn regarding the suitability of surfactants in different applications.
Download the case study to read more
Learn how QSense QCM-D reveals protein–surface interactions and adds interface-focused insight to biopharmaceutical formulation and stability work
Learn how QSense sensors enable application‑relevant biointerface interaction analysis and explore our sensor offering for different areas
Learn how QSense QCM D can be used to analyze swelling of thin films, including magnitude and dynamics.
Read about how molecule-surface interaction processes such as adsorption and desorption can be analyzed with QCM-D.
Learn best practices and step-by-step methods for accurate QCM-D coating thickness measurement on QSense sensors using QSense Omni.
Compared to QCM, QCM-D measures an additional parameter, and provides more information about the system under study.
Discover how QCM-D analysis reveals real-time etching dynamics, helping optimize cleaning processes and protect surfaces from unwanted damage.
Discover how QSense QCM-D helps tackle fouling challenges across industries
Discover how QCM-D enables real-time, label-free analysis of supported lipid membrane formation, structure, and dynamics for advanced research
Learn how QSense QCM-D analysis can reveal membrane fouling dynamics and optimize cleaning strategies for more efficient water treatment
Learn how QSense QCM-D helps detect and prevent surface-induced instabilities in biologics. Join our webinar for insights and practical examples.
Gabriel Ohlsson, Ph.D., is a former employee at Biolin Scientific where he initially held a position as an application scientist and later as a sales manager. Dr. Ohlsson did his Ph.D. in engineering physics and has spent a lot of time developing sensing technologies for soft matter material applications. One of his main tools during this research has been the QCM-D technology.
