In many practical systems, there are liquids and with that air-liquid or liquid-liquid interfaces involved. Whether it’s an air-liquid or liquid-liquid interface, the interface needs to be considered to fully describe the system at hand. When surface-active molecules are added into the mixture, the system becomes even more complex. An understanding of the mechanical behavior of soluble adsorption layers or insoluble monolayers of surface-active molecules is fundamental in many industrial areas.
Interfacial rheology studies the flow properties of liquid interfaces. The interface can be that of liquid and air or a liquid-liquid interface. Liquid-liquid interface forms when two liquids that don’t mix are in contact with each other, such as oil and water.
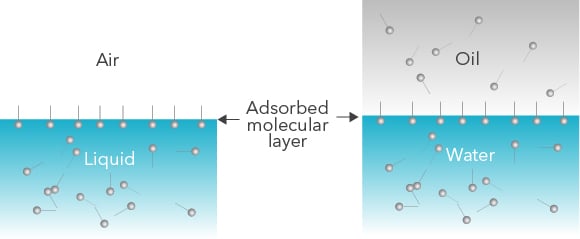
Pure liquid interfaces don’t have rheological properties but when surface-active agents are present, they form a layer at the interface that will deform when stress is applied to it.
Surface active agents are molecules that have hydrophilic and hydrophobic parts. They orient themselves at air-liquid (or liquid-liquid) interface so that the hydrophilic part is in the aqueous phase and hydrophobic part in the air (or oil phase). Surface - active agents can be polymers, proteins, and even nanoparticles.
Interfacial rheology can predict the stability of the interfaces. It’s studied by varying the size of the interface or by deforming it. The response caused by external stress is measured.
Consider a typical emulsion between oil and water, mayonnaise, for example. Mayonnaise is an oil-in-water (O/W) emulsion where oil drops are dispersed in the continuous water phase. At the oil-water interface, there is an emulsifier, a protein coming from egg yolk plasma.
To keep the properties of mayonnaise, the emulsion needs to be stable. Coalescence is one of the phenomena that destabilize emulsions. There, small droplets merge forming a larger droplet. This occurs when droplets come in contact with each other and the interfacial film is ruptured. This will eventually lead to phase separation and a ruined mayonnaise. The interfacial rheological properties of the interfacial layer will give indications on how well the interface can resist coalescence.
Apart from the food industry, interfaces stabilized by different kinds of surface-active molecules can be found on almost all industrial applications from the oil industry to biosciences.
To read more examples where interfacial rheology is important, please download the overview below.
Emulsions are dispersed systems of two immiscible liquids such as oil and water. Interfacial rheology measurements predict emulsion and foam stability.
The same measurement modes used in bulk rheology are also meaningful in interfacial rheology.
Pickering emulsions utilize solid particles to stabilize the interface between the two immiscible liquids
International Congress on Interfacial Rheology was held in Athens from the 29th of July to the 4th of August 2023.
Foam stability refers to the ability of a foam to maintain its structure and resist collapse over time.
In this blog post, the most common interfacial shear rheology methods are reviewed.
Interfacial rheology studies the response of the interfacial layer to the external stimuli at air-liquid or liquid-liquid interfaces.
Interfacial shear rheology at the gas-liquid or liquid-liquid interface is relevant in a wide range of applications where foams and emulsions are used.
Interfacial rheology is a special branch of rheology that involves studying the unique two-dimensional systems formed at interfaces.
