
Complex fluid/fluid systems, such as emulsions, gels and various surfactant solutions, are the basis of most of our everyday consumer products from detergents to healthcare, but also found in biology and industrial processes such as in enhanced oil recovery and mineral processing.Typically these complex systems include surface active constituents that creates adsorbed layer on the fluid-fluid interfaces. Properties of this adsorbed layer are important to define quality of the final product.
For example if your mayonnaise will stay as a stabile emulsion over time or how comfortable contact lenses are after a long day. Interfacial rheology is a technology to study properties of these adsorbed interfacial layers. Below you can find some typical application examples of the adsorbed interfacial layers.
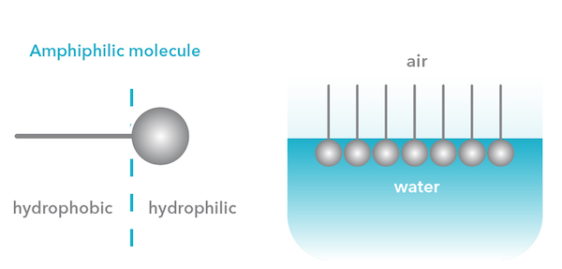
 Surfactants and surface-active polymers are commonly used to stabilize emulsions and foams in, for example, the food and cosmetics industries. Surfactants have an amphiphilic character, which means that they consist of hydrophilic and hydrophobic parts. In the adsorption process, the surfactant molecules orientate themselves so that the hydrophilic head is in water and the hydrophobic head is in oil or vapor as shown in the image below. These surface-active are preset in your everyday products constituents to form for example long lasting whipped cream.
Surfactants and surface-active polymers are commonly used to stabilize emulsions and foams in, for example, the food and cosmetics industries. Surfactants have an amphiphilic character, which means that they consist of hydrophilic and hydrophobic parts. In the adsorption process, the surfactant molecules orientate themselves so that the hydrophilic head is in water and the hydrophobic head is in oil or vapor as shown in the image below. These surface-active are preset in your everyday products constituents to form for example long lasting whipped cream.
The complex interfaces are also found in biology, for example in tear film and lung surfactants. Natural tears keep our eyes comfortable. It has been found that most of the contact lens discomfort issues are caused by the fact that the tear film breaks up. Recently it has been found that the lipid layer, an oily coating on the surface of the tear film, has viscoelastic properties that allow them to stretch and support the watery layer beneath them. The lipid layer also prevents the tear film from evaporating away. Learn more about this from this publication.
The lung surfactants have a vital function in making the process of breathing easy. During inhalation, the surfactant reduces the surface tension of tissue by a factor of about 15 making it much easier to inflate the alveoli. For some newborns, the production of natural pulmonary lung surfactant is weakened requiring the dosing of synthetic surfactant solution. Stability and viscoelasticity of the pulmonary surfactant interface is vital for its efficient functionality. Learn more about lung surfactants from KSV NIMA Application Note #8.
Want to learn more? Watch this recorded webinar with guest speaker Professor Gerald Fuller, Fletcher Jones II professor of Chemical Engineering at Stanford University.
Emulsions are dispersed systems of two immiscible liquids such as oil and water. Interfacial rheology measurements predict emulsion and foam stability.
The same measurement modes used in bulk rheology are also meaningful in interfacial rheology.
Pickering emulsions utilize solid particles to stabilize the interface between the two immiscible liquids
International Congress on Interfacial Rheology was held in Athens from the 29th of July to the 4th of August 2023.
Foam stability refers to the ability of a foam to maintain its structure and resist collapse over time.
In this blog post, the most common interfacial shear rheology methods are reviewed.
