How nanomaterials interact with the environment after they have been disposed of has many implications for potential toxicity and health concerns. Whether nanomaterials are being incorporated into commercial goods for their anti-microbial properties such as in work-out clothes or used for targeted drug therapies their overall prevalence is increasing. Therefore, the likelihood of someone coming into contact with these materials is also increasing.
QCM-D (Quartz crystal microbalance with dissipation monitoring) can be used as a step in better understanding the interactions of nanoparticles with cells by approximating the cell surface with a supported lipid bilayer (SLB) and then flowing nanoparticles across the model cell membrane to probe the nanoparticle-cell membrane interaction. Using this cell membrane mimic allows the researcher to simplify the cell, and to investigate specific aspects of the membrane by building up the complexity. A multitude of variables from either the standpoint of the model cell membrane or the nanoparticle itself can be investigated. These include:
In this post I will briefly give an introduction to this research area and highlight some key references and exciting recent work.
One of the great things about this kind of research is that the assay design details are already outlined, and there are published recipes on how to form a supported lipid bilayer onto a QCM-D sensor.
The first paper illustrating how QCM-D can uniquely probe lipid vesicle deposition and the mechanism by which the vesicles can rupture to form a SLB onto a solid substrate was written by “Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance” Keller C. A.; Kasemo B. Biophys J. 1998, 75(3), 1397-1402. In this reference, three different surfaces were investigated:
On the (1) hydrophobic surface, a monolayer of lipid was found to deposit. On the (3) gold surface, the vesicles adsorbed intact. Yet on the (2) silicon dioxide surface, the vesicles initially adsorbed intact until a significant surface concentration was reached and then the vesicles ruptured to form a lipid bilayer.
Several years later a description of the step by step protocols for how to form SLBs onto different surfaces including silicon dioxide, titanium dioxide, and gold were detailed in “Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates”Cho, NJ; Frank, C. W.; Kasemo, B.; Höök, F. Nature Protocols 2010, 5, 1096–1106. This reference is extremely useful for those new to lipid bilayers that are looking for detailed step by step instructions for how to prepare these kinds of surfaces.
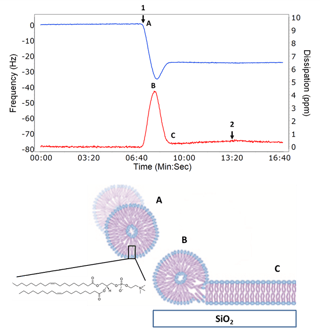 Example QCM-D data, demonstrating lipid bilayer formation, along with a corresponding illustration, is shown in the figure to the right.
Example QCM-D data, demonstrating lipid bilayer formation, along with a corresponding illustration, is shown in the figure to the right.
"The vesicle rupture to lipid bilayer “fingerprint” is unique to the QCM-D technology"
The key feature of QCM-D data when making a SLB is the characteristic rupture step shown at (B) in the figure. After a critical coverage of surface adsorbed vesicles is reached, or when an external stimulus is applied such as a reactive peptide or a change in osmotic pressure, the frequency increases as the mass of associated solvent inside the vesicle is lost as the vesicle ruptures and a lipid bilayer forms. This is also why the dissipation decreases, because the adsorbed layer is going from a soft viscoelastic vesicle film with quite a bit of associated solvent to a rigid lipid bilayer with little to no associated solvent. This vesicle rupture to lipid bilayer “fingerprint” is unique to the QCM-D technology and allows probing the mechanism for bilayer formation. Surface support composition, ionic strength, pH, vesicle complexity, and constituents such as cholesterol or membrane proteins can be altered and their effects probed on how the resulting lipid bilayer forms.
After the lipid bilayer (or intact vesicle layer) is prepared then usually a particle solution is flown across the top and the resulting signal changes correspond to how the particle interacts with the membrane surface. The effects of particle type, size, concentration, outer coating chemistry, solution ionic strength, pH, and temperature can all be measured. A number of different groups are working in this area. Below I list five different studies:
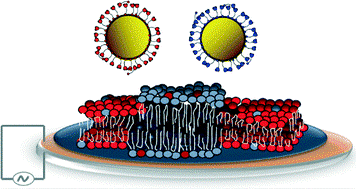 Open access “Formation of supported lipid bilayers containing phase-segregated domains and their interaction with gold nanoparticles” E. S. Melby, et al., Environ. Sci.: Nano2016, 3, 45-55. Published by The Royal Society of Chemistry..
Open access “Formation of supported lipid bilayers containing phase-segregated domains and their interaction with gold nanoparticles” E. S. Melby, et al., Environ. Sci.: Nano2016, 3, 45-55. Published by The Royal Society of Chemistry..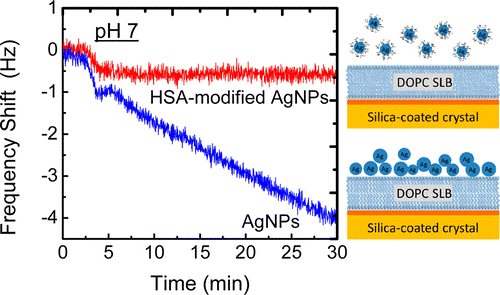 Published with permission from “Influence of Solution Chemistry and Soft Protein Coronas on the Interactions of Silver Nanoparticles with Model Biological Membranes” Q. Wang, et al., Environ. Sci. Technol. 2016, 50 (5), 2301–2309. Copyright 2016 American Chemical Society.”
Published with permission from “Influence of Solution Chemistry and Soft Protein Coronas on the Interactions of Silver Nanoparticles with Model Biological Membranes” Q. Wang, et al., Environ. Sci. Technol. 2016, 50 (5), 2301–2309. Copyright 2016 American Chemical Society.”
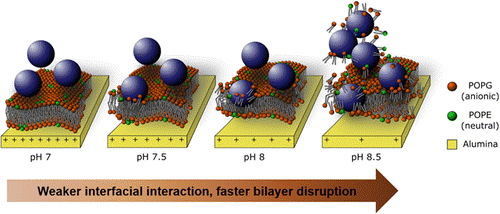
“TiO2 nanoparticle interactions with supported lipid membranes – an example of removal of membrane patches” F. Zhao, et. al., RSC Adv., 2016, 6, 91102-91110.
Read more about the assessment and characterization of the interaction of nanomaterials with biological systems in our application note:
Review of nanoparticles interacting with the environment/humans
Discover how QCM-D enables real-time, label-free analysis of supported lipid membrane formation, structure, and dynamics for advanced research
QCM-D was used to compare the potency and mechanisms of action of two different detergents in disrupting lipid membranes
Read about Prof. Jackman's experience using QCM-D to study surfactant-interaction with model membranes
Read about Prof. Jackman's experience using QCM-D in the field of membrane biophysics.
Watch the webinar to learn more about how to combine QCM-D and Neutron reflectrometry to examine membrane biochemistry at the solid-liquid Interface
Read about two cases where QSense QCM-D technology was used to explore viral membrane disruption and an antiviral strategy towards pandemic preparedness.
