Single-cell trapping means spatially isolating single cells from a cell suspension. This is the first step for single-cell analysis, which means analyzing e.g. the metabolomic or transcriptomic contents of individual cells. There are several ways to achieve single-cell trapping but one way is to utilize wettability patterned surfaces with alternating superhydrophobic and -philic areas.
The defining feature of a superhydrophobic surface is the extremely low water adhesion on it. This is most commonly characterized by either contact angle goniometry (both advancing and receding contact angles are very high and the hysteresis is very low) or by sliding angle measurements (sliding angles are very low). On a smooth solid surface, the highest contact angles are typically observed on fluorinated surface chemistries but even these are only around 120°, which is not enough for superhydrophobicity. The reason for this is the van-der-Waals interactions between the surface and water. Superhydrophobicity is based on a topography trick: a rough surface with hydrophobic surface chemistry leaves behind an air-solid composite interface since capillary pressure prevents the roughness to be fully wetted. This air fraction of the composite interface has much lower interactions with water than the solid part, therefore leading to higher contact angles, less adhesion, and superhydrophobic surfaces. The air fraction can easily be >90%.
This air fraction of the composite interface has much lower interactions with water than the solid part, therefore leading to higher contact angles, less adhesion, and superhydrophobic surfaces. The air fraction can easily be >90%.
Superhydrophilic surfaces are in principle much easier since many highly polar or charged surface chemistries already have close to 0° advancing contact angles. These extremely high-energy surfaces have a drawback in that they are often quite unstable, for example, due to their high potential to adsorb contaminants from the atmosphere. Roughness can be used to enhance the wettability of inherently slightly less hydrophilic surface chemistries and superhydrophilic surfaces made with this technique can be more stable.
Wettability depends on both the topography and the surface chemistry. Therefore, to create patterned areas of different wettability one has three choices: 1. pattern the topography, 2. pattern the chemistry, and 3. pattern both, either separately or at the same time. Of these, the first choice of patterning only the topography tends to be limited in the range of contact angles achievable, whereas patterning the chemistry, especially on top of rough topography, can give the entire range from 0° to close to 180°. The patterning methods are many, including photolithography, laser processing, and printing techniques.
In a recent publication [1], we utilized an array of superhydrophilic microdots on a superhydrophobic background to capture single cells from the cell suspension. 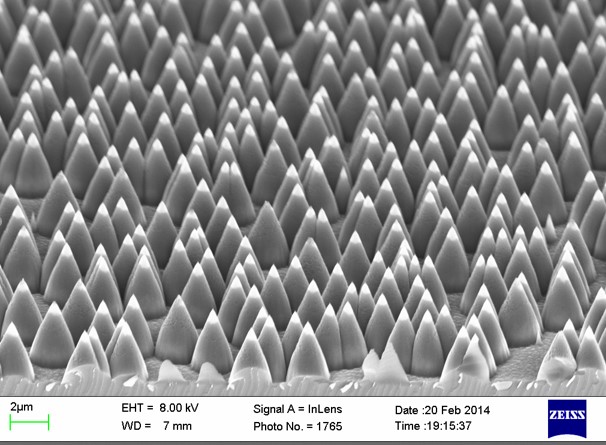 For this application, we needed 10 µm resolution, extreme superhydrophobicity, and modest superhydrophilicity. Our solution was to use black silicon nanograss coated by a fluoropolymer thin film as the superhydrophobic surface, and a smooth silicon surface hydrophilized by oxygen plasma as the superhydrophilic surface. Both the topography patterning and the surface chemistry patterning were done by optical lithography (a resist layer masked first the nanograss formation and later the oxygen plasma treatment).
For this application, we needed 10 µm resolution, extreme superhydrophobicity, and modest superhydrophilicity. Our solution was to use black silicon nanograss coated by a fluoropolymer thin film as the superhydrophobic surface, and a smooth silicon surface hydrophilized by oxygen plasma as the superhydrophilic surface. Both the topography patterning and the surface chemistry patterning were done by optical lithography (a resist layer masked first the nanograss formation and later the oxygen plasma treatment).
Single-cell trapping can be based on Poisson dilution (handling suitably low volumes and concentration that statistically only one cell is present) or on size exclusion (utilizing structures with such dimensions that only a single cell can fit into a trap). In either case, the methods are usually based on microfluidics and microtechnology, as this allows both size exclusion and large-scale parallelization which is needed for trapping typically 1000s of cells.
The trapping method in our work [1] is based on a cell suspension droplet that is dragged across the superhydrophilic/superhydrophobic array. It leaves behind small droplets only on the superhydrophilic areas. The size of these droplets is optimized so that two cells cannot fit inside. This mechanism utilizes the basic properties of superhydrophilic and superhydrophobic surfaces. The adhesion of water onto the superhydrophilic spots is high enough to leave behind the droplet even if this means creating the new water-air interface. On the other hand, the adhesion of water onto the superhydrophilic areas is so low that we get individual and isolated droplets and no stray droplets outside the intended array.
With this method, we were able to trap and isolate human peripheral blood mononuclear cells. Our optimized designs had 28 000 spots /cm2 and we were able to achieve 10% - 30% single-cell occupancy rates with only a roughly 1% of the spots containing more than one cell.
To hear more about single-cell trapping and how wettability can be utilized to achieve that, watch the webinar below.
[1] P. Raittinen, P. Elomaa, P. Saavalainen, and V. Jokinen, "Single cell trapping by superhydrophobic/superhydrophilic microarrays", Advanced materials interfaces 8 (2021) 2100147.
Understand how superhydrophobic surfaces are defined, how they work, and how to measure them using contact angle methods. Explore key industrial applications—from self‑cleaning and anti‑icing to drag reduction—and what to consider when evaluating durability.
Advancing and receding angles should be measured as the low contact angle hysteresis is also a requirement for superhydrophobicity.
There are three contact angle measurement methods for superhydrophobic surfaces; static, advancing/receding and roll-off angle.
A self-cleaning surface is any surface with the ability to readily remove any dirt or bacteria on it. Self-cleaning surfaces can be divided into three different categories; superhydrophilic, photocatalytic and superhydrophobic.
Blood-repellent surfaces are needed in medical devices that come in contact with blood. The traditional approach has been the use of antithrombotic surface treatments However, these coatings are prone to eventually wear-off. Superhydrophobic surfaces have been proposed as an alternative solution.
With increasing understanding of the superhydrophobicity, the measurement methods to quantify the degree of hydrophobicity deserve some thought.
This video will explain two main methods for measuring dynamic contact angle.
Superhydrophobic surfaces were an instant hit in the scientific community when they were introduced over two decades ago
Ville Jokinen (Ph.D) is a lecturer at Aalto University, School of Chemical technology. His research interests are microfabrication, wetting, microfluidics and superhydrophobicity and their chemical and biological applications.
