
Polymers and polyelectrolytes of various conformations are used in many applications where there is a need to tailor the interfacial properties to promote a certain interaction with the surrounding environment. Here we show how polymer layer crosslinking and collapse can be characterized.
To tailor the interfacial properties of these layers, it is important to characterize and understand the conformational behavior, such as the degree of hydration and transitions from a hydrated to a collapsed or crosslinked state, and vice versa, Figure 1. The swelling and collapse of polymer brushes, and other thin films, can be characterized by QCM-D and other technologies, which will sense the water uptake and water release as changes in mass.
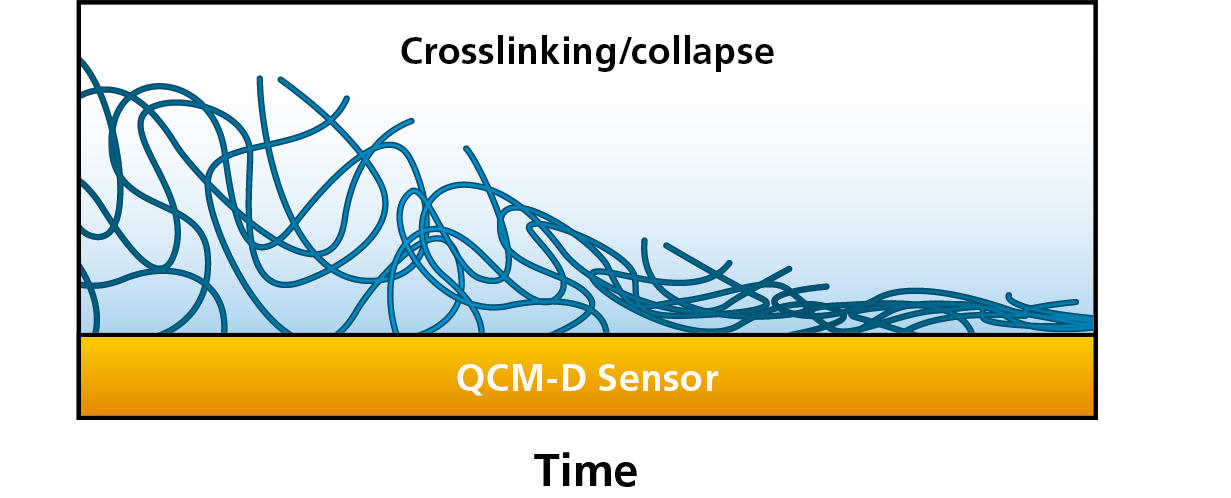
Figure 1. From left to right, this schematic illustration shows how a thick and hydrated film releases water and collapses into a thin layer at the surface.
As an example, let’s look at the transitions between hydrated and dehydrated states of polymer brushes made of chitosan. At low pH, the brush will be in a hydrated state, while at high pH it will be in a dehydrated state. It is also possible to use anions for crosslinking of the brush.
First, the QCM-D sensor is coated with the polymer. Next, the chitosan polymer brush layer is exposed to solutions of different pH and counter-anion type and the effect on the film thickness is monitored.
The results, Fig. 2., show how the thickness of the polymer varies as it goes through the swelling and collapse transformation when the pH and counter-anion are varied. It is also interesting to note that when the citrate anions are replacing the acetate anions (similar pH, step 2 in the figure), ionic cross-links are formed, which results in a collapse of brush layer.
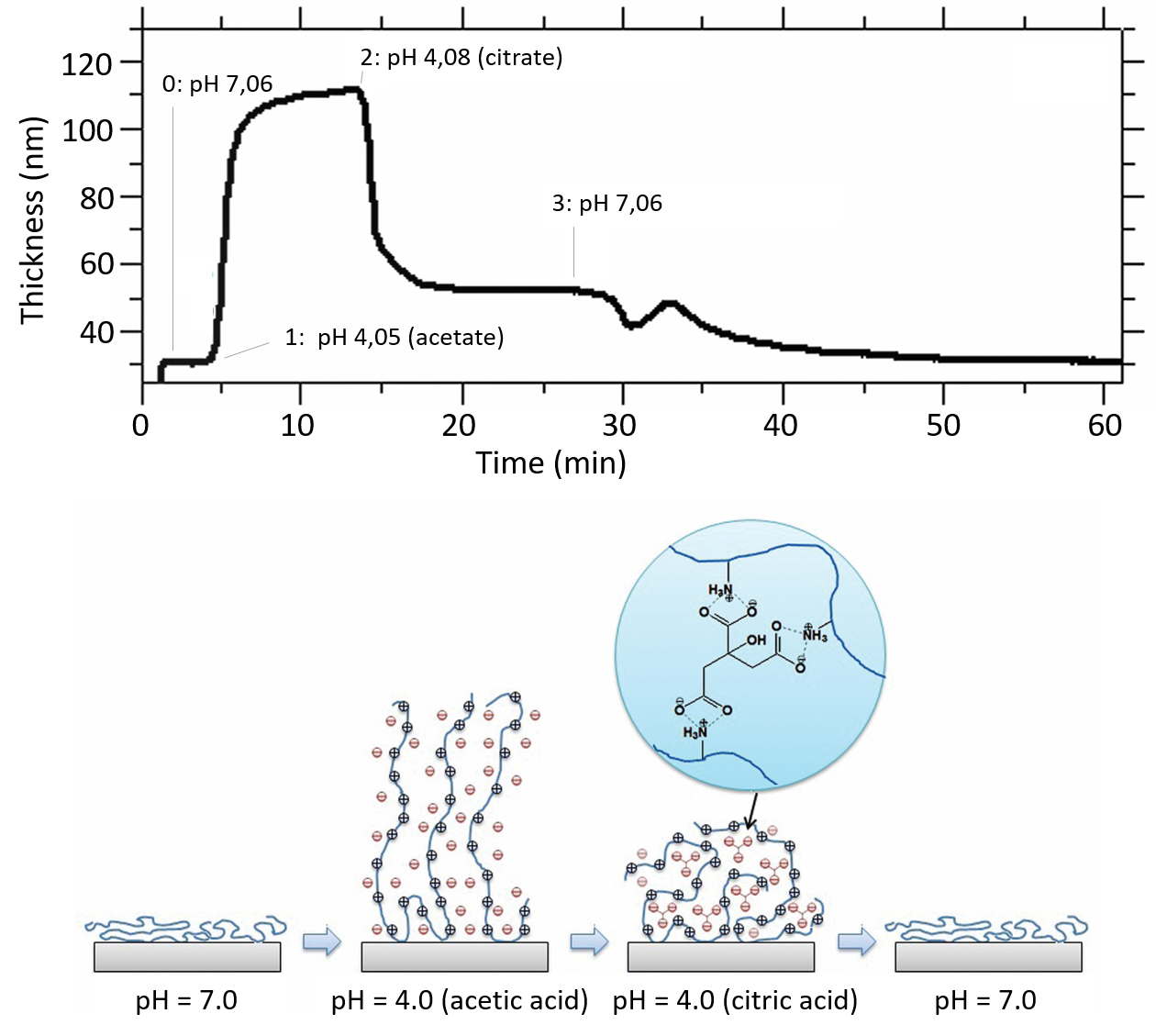
Figure 21. (Top) The thickness of the Chitosan brush layers when exposed to different solution pH and counter-ions. (Bottom) Schematic illustration of the structure of the chitosan brush layer as a function of pH and counter-ion type.
Polymer brushes, multilayers and hydrogels, i.e. cross-linked polymer networks, are all, more or less, hydrated and viscoelastic, depending on the molecular conformation at the interface. The conformation will have a major impact on the resulting interfacial properties and will influence the interaction with the ambient such as e.g. protein adsorption or prevention of bacteria adhesion. The conformation of the polymer layer can be characterized with for example QCM-D, which also enables straightforward detection of polymer brush transitions between hydrated and non-hydrated states.
Download the overview to read more about what information you can obtain with QSense QCM-D.
1. H-S Lee, et al., J. Mater. Chem., 22, 19605, 2012
Learn how QSense QCM-D reveals protein–surface interactions and adds interface-focused insight to biopharmaceutical formulation and stability work
Learn how QSense sensors enable application‑relevant biointerface interaction analysis and explore our sensor offering for different areas
Learn how QSense QCM D can be used to analyze swelling of thin films, including magnitude and dynamics.
Read about how molecule-surface interaction processes such as adsorption and desorption can be analyzed with QCM-D.
Learn best practices and step-by-step methods for accurate QCM-D coating thickness measurement on QSense sensors using QSense Omni.
Compared to QCM, QCM-D measures an additional parameter, and provides more information about the system under study.
Discover how QCM-D analysis reveals real-time etching dynamics, helping optimize cleaning processes and protect surfaces from unwanted damage.
Discover how QSense QCM-D helps tackle fouling challenges across industries
Discover how QCM-D enables real-time, label-free analysis of supported lipid membrane formation, structure, and dynamics for advanced research
Learn how QSense QCM-D analysis can reveal membrane fouling dynamics and optimize cleaning strategies for more efficient water treatment
Learn how QSense QCM-D helps detect and prevent surface-induced instabilities in biologics. Join our webinar for insights and practical examples.
Gabriel Ohlsson, Ph.D., is a former employee at Biolin Scientific where he initially held a position as an application scientist and later as a sales manager. Dr. Ohlsson did his Ph.D. in engineering physics and has spent a lot of time developing sensing technologies for soft matter material applications. One of his main tools during this research has been the QCM-D technology.
