In the previous posts of our series of blog posts on different contact angles, we have already discussed static and roughness corrected contact angles. Both are often used to calculate the surface free energies of the solid. However, while roughness corrected contact angle can be considered to be slightly closer to the equilibrium values, the static angle contact angle cannot typically be considered the most stable contact angle on a given surface. Due to this, a lot of criticism has been presented against static contact angle measurements, and instead, measurement of both advancing and receding contact angles is highly encouraged. In this blog post, we are discussing the measurement and usage of the advancing contact angle, while the receding contact angle is the topic of the next blog post.
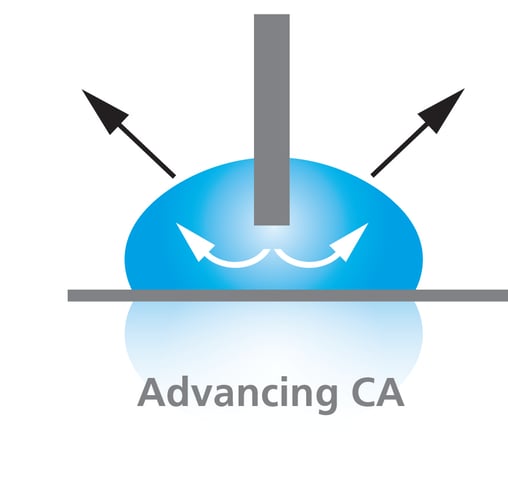
Advancing contact angle is the highest possible contact angle measured on a previously unwetted solid surface.
Advancing contact angles are measured when the liquid front is advancing over the surface to be measured. This can be done in different ways with an optical tensiometer such as tilting the sample or increasing the volume of the droplet while at the same time recording the contact angle values. Advancing contact angles are also measured with force tensiometers with the Wilhelmy plate method by immersing the sample into the liquid and measuring a force by extremely sensitive balance. Washburn method, which is primarily used for powders, is also measuring the advancing contact angle.
On a chemically heterogenous surface, the advancing angle is more sensitive to the low-energy components i.e. apolar parts of the surface. If advancing water contact angle is measured on a primarily hydrophobic surface, the hydrophilic inclusions from the surface will not affect the advancing contact angle value until they cover a significant portion of the hydrophobic surface. On a hydrophilic surface, the advancing water contact angle quickly rises to a high value even if the surface is only slightly covered with the hydrophobic inclusions. The value is also not much affected even with an increasing fraction of the hydrophobic components.
The measurement of advancing is often recommended as it provides more reproducible contact angles compared to static (and receding) values. The advancing contact angle is typically closer to the equilibrium value than the receding one but cannot still be used as an equivalent value. However, there are situations where the advancing contact angle describes the problem better than other contact angles. For example, the capillary rise is driven by the advancing contact angle. Capillary rise is an important phenomenon in microfluidics and also place a role in the wettability of powders. Advancing contact angle is also more important in applications where spreading phenomena are important, such as inkjet printing, spray coating, and pesticide deposition.
Learn how to use contact angle measurements to evaluate surface cleanliness on different materials and link cleaning steps to reliable adhesion and coating quality.
Learn how wettability, surface free energy and surface roughness define paper and paperboard surface properties – and how to measure them for reliable print, coating and sealing performance.
Smart materials are materials that sense a change in their environment and respond in a useful, reversible way.
Discover how contact angle measurements help optimize plasma treatment for improved wettability and adhesion in industrial applications.
Discover why contact angle is essential for adhesion, coatings, and quality control. Learn how surface wettability impacts product performance.
Discover why PFAS-free coatings are needed, the challenges they present, and key strategies for developing high-performance alternatives.
At the heart of droplet formation are two key molecular forces: cohesion and adhesion.
Contact angle measurements provide a golden standard for evaluation of surface properties for quality control.
Contact angle is the angle a droplet forms in contact with a solid surface. Thermodynamically, it is a balance between cohesive and adhesive forces.
