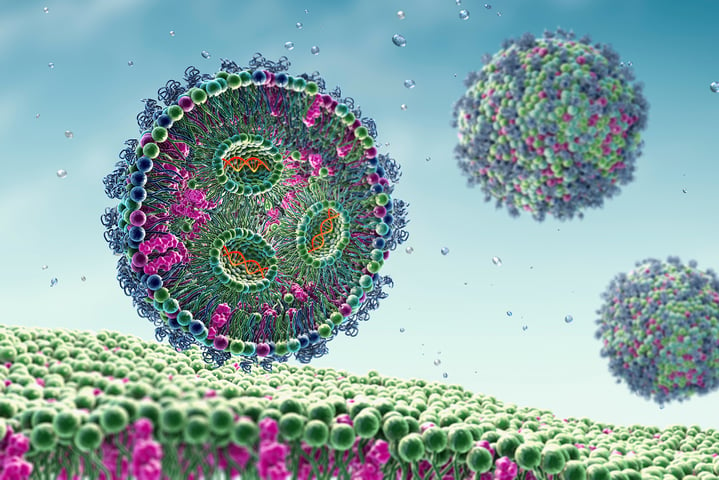
Lipid nanoparticle-based drug delivery is a fascinating concept which has received a lot of attention since the release of the COVID-19 mRNA vaccines. Intrigued, and wanting to learn more about lipid nanoparticle designs for drug delivery purposes and related areas, I got the opportunity to talk to Prof. Fredrik Höök, Professor of Nano and Biophysics, at the Department of Physics at Chalmers University of Technology who works in this scientific area.
We started this work 4- 5 years before the pandemic, Prof. Höök says. Already then, pharmaceutical companies were interested in the concept of RNA delivery. About 90% of what our research group does, is using surface analytical tools for studies of biointerface processes. The past couple of years, we have been very focused on investigations of biological nanoparticles, ranging from viruses and how viruses interact with mimics of cell surfaces, and extracellular vesicles, which could be described as cell-released biological nanoparticles, addressing similar questions.
When it comes to extracellular vesicles, we also characterize their properties, Prof. Höök continues. Extracellular vesicles are fairly heterogenous, which makes characterization complicated when it comes to some laboratory techniques like surface plasmon resonance or QCM. So, there we have worked a lot with high resolution microscopy, both label-free and fluorescently labeled based methods. That knowledge has led to a recent interest in lipid nanoparticle design for drug delivery purposes. We are not working with vaccine development per se, but the lipid nanoparticle formulations share significant similarities with those vaccines that have, or so far seem to have, saved many of us through the pandemic. That is a short summary of what we do, Prof. Höök says.
Formerly, I am the director, but practically I am also a coordinator of activities in the industrial research center FoRmulaEx, Prof. Höök says. The center originates from a call from the Swedish strategic research Foundation, SSF, where they asked industries to approach academia with questions which they could not work on directly, but where they foresee a need of academic research. The academic groups cover medical- and biological competence as well as competence in organic synthesis, bio-imaging and biological nanoparticle analytics. My group represents the last piece of that cake. So, I have a small activity in the center, but it is much broader than my share of course, Prof. Höök says.
The mRNA vaccines that have been launched successfully during the COVID-19 pandemic are based on a type of lipid nanoparticle formulation which encapsulates the mRNA and acts as a drug carrier, Prof. Höök explains. The truth is, however, that the efficiency of the mRNA delivery all the way into the cell, where the mRNA is converted into the protein, in the case of vaccine - the spike protein to trigger the immune response, is very inefficient. It is only on the order of 1-2% of the mRNA injected. If we could increase that efficiency of delivery, we could reduce the dose, Prof. Höök says.
The RNA is also fulfilling the purpose of triggering an immune response, he continues. I.e., in the case of vaccines, the high dose triggers an immune reaction which is very helpful in this situation. So the low efficiency is not so much of a problem. But if you would like to use this principle to treat other diseases, such as diabetes, cardiovascular diseases and so forth, we need to increase the efficiency because in these situations, when we would be receiving the medication more often, for example once a week or once a month, we cannot accept having the type of reactions that we have when we are injected with a vaccine. An increased efficiency is not an unrealistic goal, and there is a lot of effort into that, Prof. Höök says.
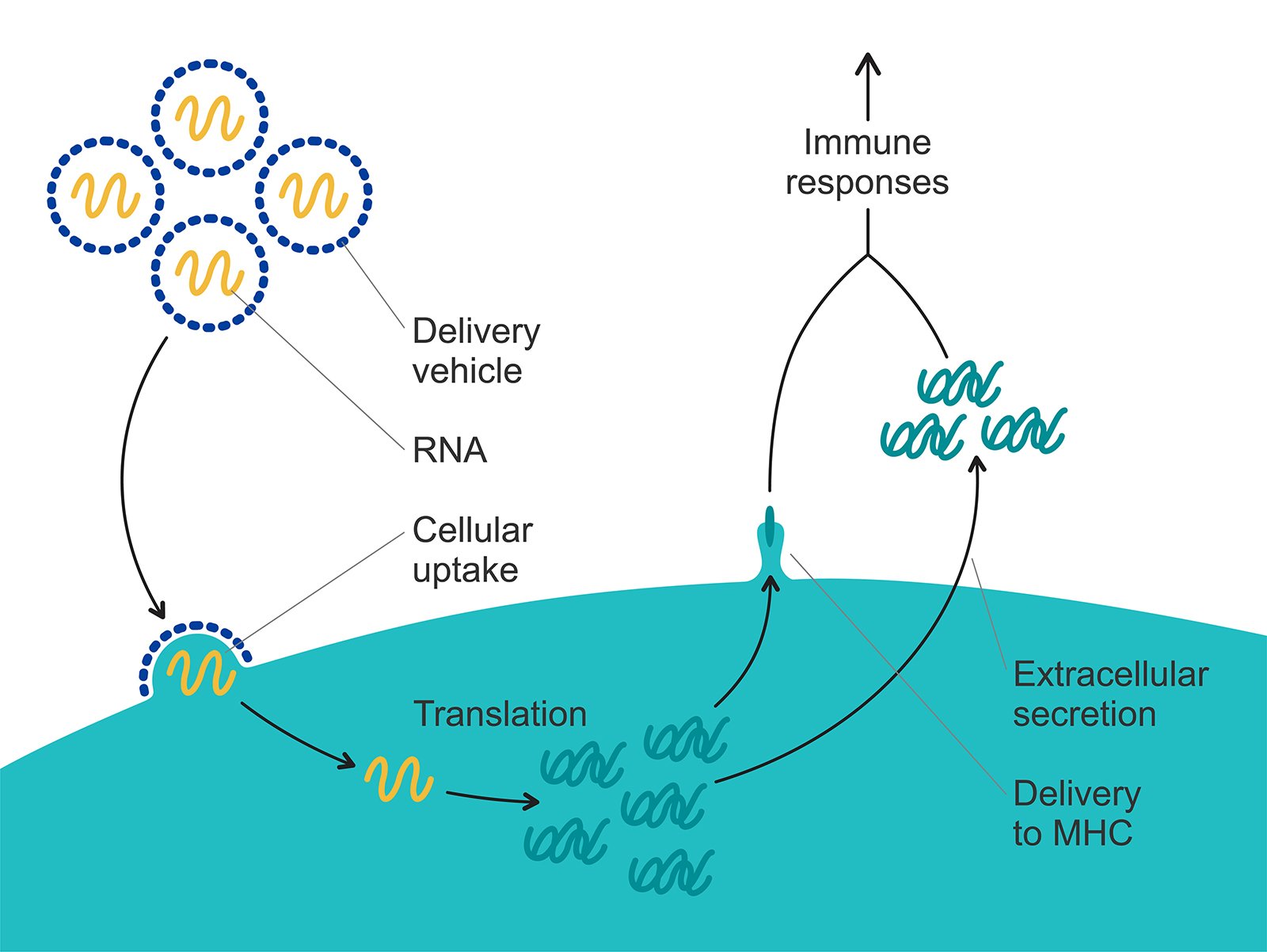
Figure 1. The lipid nanoparticle formulation encapsulates the mRNA and acts as a drug carrier. The mRNA is delivered all the way into the cell, where it is converted into the spike protein which triggers the immune response.
There are a few critical components when it comes to the uptake of lipid nanoparticles into the cell, and we are working on three of these, Prof. Höök says.
One aspect that we are looking into is what happens to the lipid nanoparticle once it is injected and comes in contact with serum. The first thing that happens is that protein and biomolecules in the serum and blood coat the lipid nanoparticle and form a so-called corona. This is a surface reaction, and we are experts in looking at surface reactions, Prof. Höök says. This process dictates the efficiency by which the lipid nanoparticles are taken up by cells, and also, to some extent, which cells that that they are taken up by. The lipid nanoparticle will see thousands of different proteins, so the composition of the protein corona will vary. Each lipid nanoparticle will have a protein coronation with different composition, but there are similarities of course. Certain proteins that bind are crucial for the uptake because they are recognized by receptors at the cell surface. And therefore, the time evolution of the protein coronation of the lipid nanoparticles is one process that we are looking into.
The second component that we investigate is what structural changes that are involved in the lipid nanoparticle as protein binds during the coronation and when the pH drops, Prof. Höök continues. The actual lipid structure is crucial for the next event, which occurs once the lipid nanoparticle has been taken up by a cell, via so called endocytosis. Those structural changes are hard to inspect with surface analytical tools, at least the ones that we use. Although there are certain possibilities with neutron reflectometry. This is a is a method that we are looking into now to see whether it can help us somewhat, Prof. Höök says.
Endocytosis could be explained as the cells digestion system, Prof. Höök says. When we eat food, we digest and take care of what is useful to us and then we get rid of the rest. These cells have the same principle, and this endosomal system fulfills that purpose. What they primarily do is that they digest what is taken up. There is an enzymatic machinery and a significant drop in the pH in the endosome. This will, of course, harm the mRNA. Therefore, we need to formulate the lipid nanoparticles such that they, early on, in the endosome, undergo some kind of physicochemical change that leads to a fusion with the endosomal membrane. The trick that is used for this to happen is, as far as I am aware, not inspired by how viruses are doing, Prof. Höök says.
The lipid nanoparticles contain what is called an ionizable lipid. These ionizable lipids are not present naturally in viruses, they use other pH-induced triggers and protein-based systems for this delivery to occur, Prof. Höök explains. But, while the pH is near neutral outside the cell and in the cytosol of the cell, the pH in the endosome gradually drops from the physiological pH of 7.4 all the way down to four or five. As the pH drops, you have got a lot of hydrogen ions and they can protonate ionizable groups. So, one critical component in the lipid nanoparticle is an ionizable lipid which becomes positively charged as the pH drops. The endosomal membrane is negatively charged, and that leads to an association between the lipid nanoparticle and the endosomal membrane and even fusion between these membranes. Once fusion occurs, there is an escape of mRNA, or can be an escape of mRNA, across the endosomal membrane. And there we have another process where surfaces are involved - the surface of the lipid nanoparticle and the surface of the endosomal membrane. So, with our surface analytical tools, we generate mimics of the endosomal membrane and investigate how the pH influences the interaction between lipid nanoparticles and the endosome. And that is the third component, Prof. Höök says.
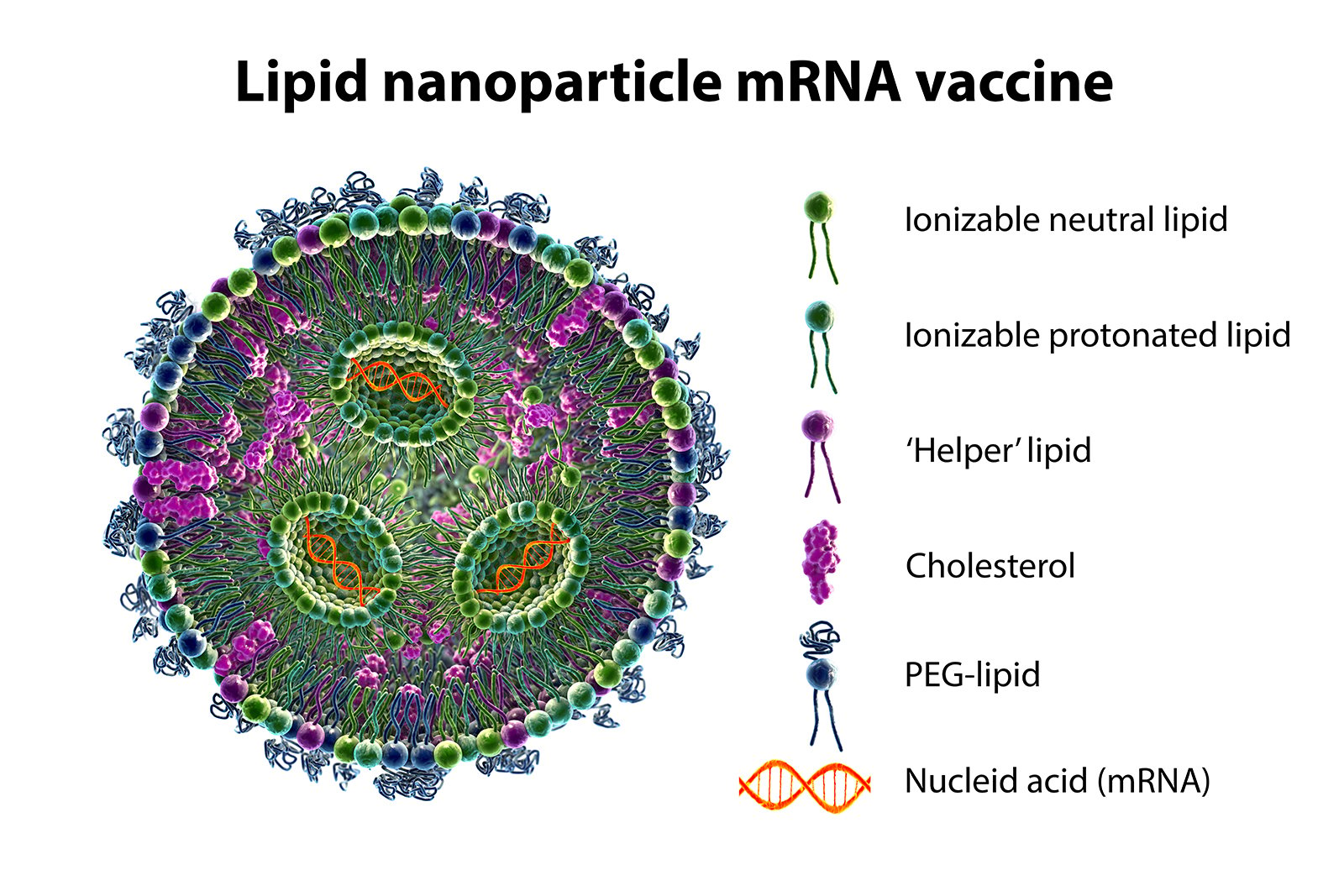
Figure 2. The lipid nanoparticle formulation is engineered to protect the cargo all the way to the critical endosomal escape event. In addition to the mRNA, the formulation includes several different types of molecules. The ionizable lipid helps package the mRNA in the carrier. There are also protective lipids, pegylated lipids, some conventional natural lipids, and cholesterol.
The lipid nanoparticle formulation is engineered to protect the cargo all the way to the critical endosomal escape event, and the ionizable lipid helps package the mRNA in the carrier, Prof Höök explains. The lipid nanoparticles are made at low pH where the ionizable lipid is positively charged, and then they associate in a microfluidic-based fabrication process with the RNA which is negatively charged. There are also protective lipids, cholesterol, pegylated lipids, and some conventional natural lipids. Together, they spontaneously form the lipid nanoparticles where the cargo is protected, preferably all the way to the critical escape event. But this is a complex process, and as I already mentioned, the efficiency is not very high. The efficiency of viruses is not 100% either. Not every virus that interacts with a cell is taken up. The efficiency is likely to be higher than we have so far reached though. And when I say ‘we’, it is really not we who developed this lipid nanoparticle design. We have learned how they are made through the collaborators in FoRmulaEx. Much of this is common knowledge, but of course there is a lot of new knowledge that is generated that is not yet public, neither for us or for anyone else, because this is this is now a very, very hot topic where we try to contribute fundamental insights that potentially, or hopefully, could aid the further development of this delivery concept, Prof. Höök says.
Even though the model membranes that we use are very reductionistic in a sense, many of the physical principles like domain formation, diffusion events and so forth, are still fairly representative, Prof. Höök says. A major limitation with supported membranes formed on glass, which is one of the few materials that the membranes form on, however is that neither the organelles nor the outer cell membrane is on a solid surface. The surface hinders the mobility of lipids, the domain formation and so forth, which is an obstacle when it comes to translating our observations to the world. There has been significant effort devoted to finding means to separate the distance between the membrane and the solid surface, for example using tethered membranes. Once those principles of separating the membrane from the surface start to be more and more established, we will also be able to increase the complexity of the membrane mimics, Prof. Höök says.
One popular route to increase the membrane complexity, which is already in use, is to do hybrid membranes, Prof. Höök continues. You take a cell membrane and mix that with a fraction of synthetic lipids that promote bilayer formation on planar surfaces. Then you mimic the complexity of the cell membrane. That direction looks promising. But what is not sustained yet is to preserve the asymmetry of the membrane, i.e., the fact that the lipid composition on one side of the membrane is different to the lipid composition on the other side. The asymmetry does not survive this process when it comes to planar membranes. You can make vesicles and there the asymmetry is more preserved. This aspect makes vesicles interesting. A drawback with vesicles, however, is that it is not as straightforward to use the surface analytical tools as it is on planar surfaces, Prof. Höök says.
The leading tools that we use in our research are QCM and surface plasmon resonance, SPR, Prof. Höök says. We do not use the conventional Biacore SPR, but spectroscopic SPR operating at more than one wavelength. The reason is that with different wavelengths you generate evanescent fields that extend more or less far out in the solution, from 100 nm to 200 - 300 nm, depending on the wavelength. This is not crucial if you look at really thin films, but if you study, for instance, tethered vesicles on the surface, the response is different depending on the wavelength that you operate with, and this allows you to make thickness determinations fairly accurate. You can also look at structural changes. Since the spectroscopic SPR allows us to determine the film thickness, we can, in addition to the mass, also see where the protein binding on the lipid nanoparticle, or vesicle, is leading to a contraction of the vesicle. The reason for that interest is that this signal reveals structural changes. And if there is something QCM is good at it is to see that there is a structural change. For rigid systems, one can fairly accurately directly from a QCM response tell what is going on. But for non-rigid, viscoelastic systems, it is very helpful to complement that with, for instance, a thickness determination. So combined the two methods provide the type of analytical input you need to strengthen your conclusions, Prof. Höök says.
I have mentioned virus particles and extracellular vesicles, Prof. Höök continues. The extracellular vesicles are not so different from viruses, but they communicate genetic information. At least they are believed to communicate genetic information between cells in a way not so different from how viruses infect, but not for the purpose of causing disease, they are rather functional vehicles. But they are also very heterogeneous. One drawback both with SPR and QCM is that they are ensemble averaging methods. So, there is an emerging realization of the importance of single nanoparticle resolution techniques. We are working with surface-based microscopy, either total internal reflection microscopy or light scattering-based microscopy, waveguide-based scattering microscopy. There is also another very interesting technique that we do not yet have in the lab, and this is interferometric scattering microscopy, iSCAT. This has tremendous sensitivity and allows you to look at individual nanoparticles. This is the third technique that we use where one can also sometimes deduce structural changes and relate that to what we see with SPR and what we see with QCM. So, I would say those are our key workhorses, Prof. Höök says.
Listen to the full interview with Prof. Fredrik Höök to learn more about his research and work related to biological nanoparticles, virus infections, and vaccine development.
Learn how QSense QCM-D reveals protein–surface interactions and adds interface-focused insight to biopharmaceutical formulation and stability work
Learn how QSense sensors enable application‑relevant biointerface interaction analysis and explore our sensor offering for different areas
Learn how QSense QCM D can be used to analyze swelling of thin films, including magnitude and dynamics.
Read about how molecule-surface interaction processes such as adsorption and desorption can be analyzed with QCM-D.
Learn best practices and step-by-step methods for accurate QCM-D coating thickness measurement on QSense sensors using QSense Omni.
Compared to QCM, QCM-D measures an additional parameter, and provides more information about the system under study.
Discover how QCM-D analysis reveals real-time etching dynamics, helping optimize cleaning processes and protect surfaces from unwanted damage.
Discover how QSense QCM-D helps tackle fouling challenges across industries
Discover how QCM-D enables real-time, label-free analysis of supported lipid membrane formation, structure, and dynamics for advanced research
Learn how QSense QCM-D analysis can reveal membrane fouling dynamics and optimize cleaning strategies for more efficient water treatment
Learn how QSense QCM-D helps detect and prevent surface-induced instabilities in biologics. Join our webinar for insights and practical examples.
Learn about the top QSense sensors for analyzing biopharmaceutical drug-surface interactions in the context of IV bags.
