Quartz Crystal Microbalance, QCM, is a surface sensitive real-time technology that can be used to monitor molecular interaction at surfaces and interfaces via detection of changes in the resonance frequency of the QCM sensor. Using the Sauerbrey equation, the frequency change can be converted to change in mass, allowing characterization of mass uptake and mass loss at the surface.
QCM technology, which is essentially a balance for very small masses, has been around since the 60’s. With a mass resolution typically in the ng/cm2 range, it enables monitoring of build-up and removal of thin and rigid layers at the sensor surface. Traditionally, it was used for monitoring of thin-film deposition in vacuum and gas phase, but in the last couple of decades the traditional QCM configuration has evolved and extended versions, such as QCM-D, now exist. This has enabled the usage of the technology to expand and spread into other applications, and the method is now commonly used for liquid-phase measurements.
The core of QCM technology is the sensor, which is traditionally made of quartz, a piezoelectric material. A thin disk of the piezoelectric material is sandwiched between two electrodes, Fig 1A. Applying an electric field over the disk, there will be a mechanical oscillation, Fig 1B. The resonance frequency depends on the sensor design and is typically 5-10MHz, but it can be both lower and higher.
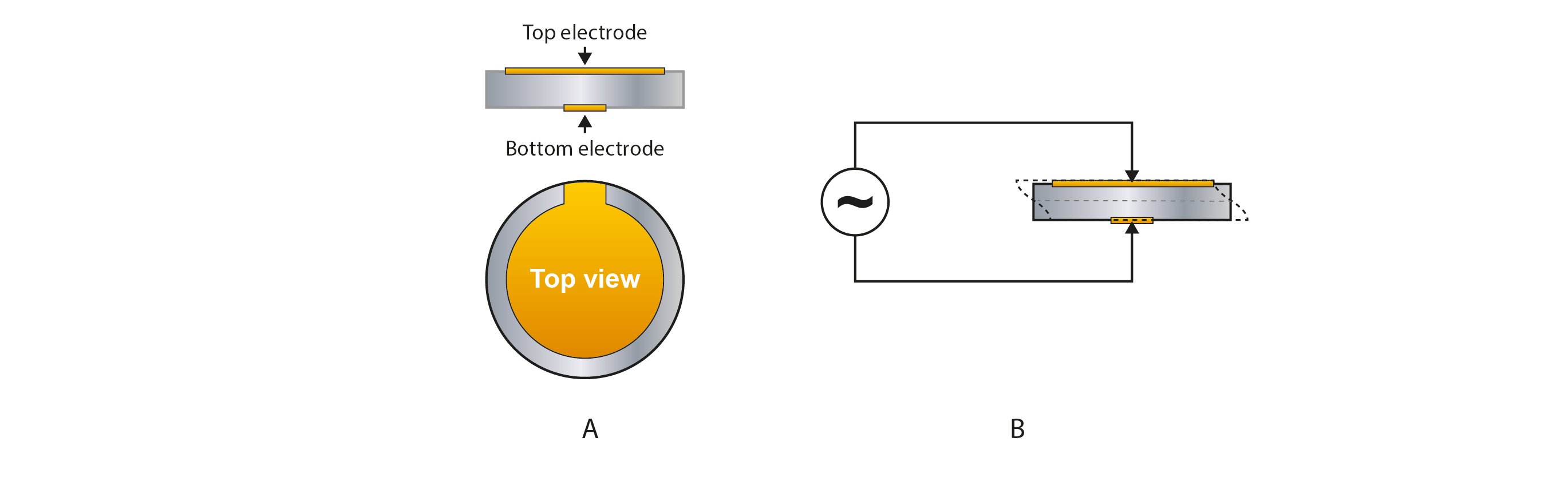
Figure 1. Schematic illustration (not to scale) of a (A) QCM sensor made of a thin disk of a piezoelectric material sandwiched between two electrodes. The disk material is typically quartz, and the electrodes are typically made of gold, but other materials can be used. In (B), an alternating voltage is applied over the sensor, inducing a mechanical oscillation of the sensor
The QCM measurement is achieved by monitoring the change in resonance frequency, Δf, when mass is added to, or removed from, the sensor surface. When mass is added, the resonance frequency decreases, Fig. 2, and vice versa.
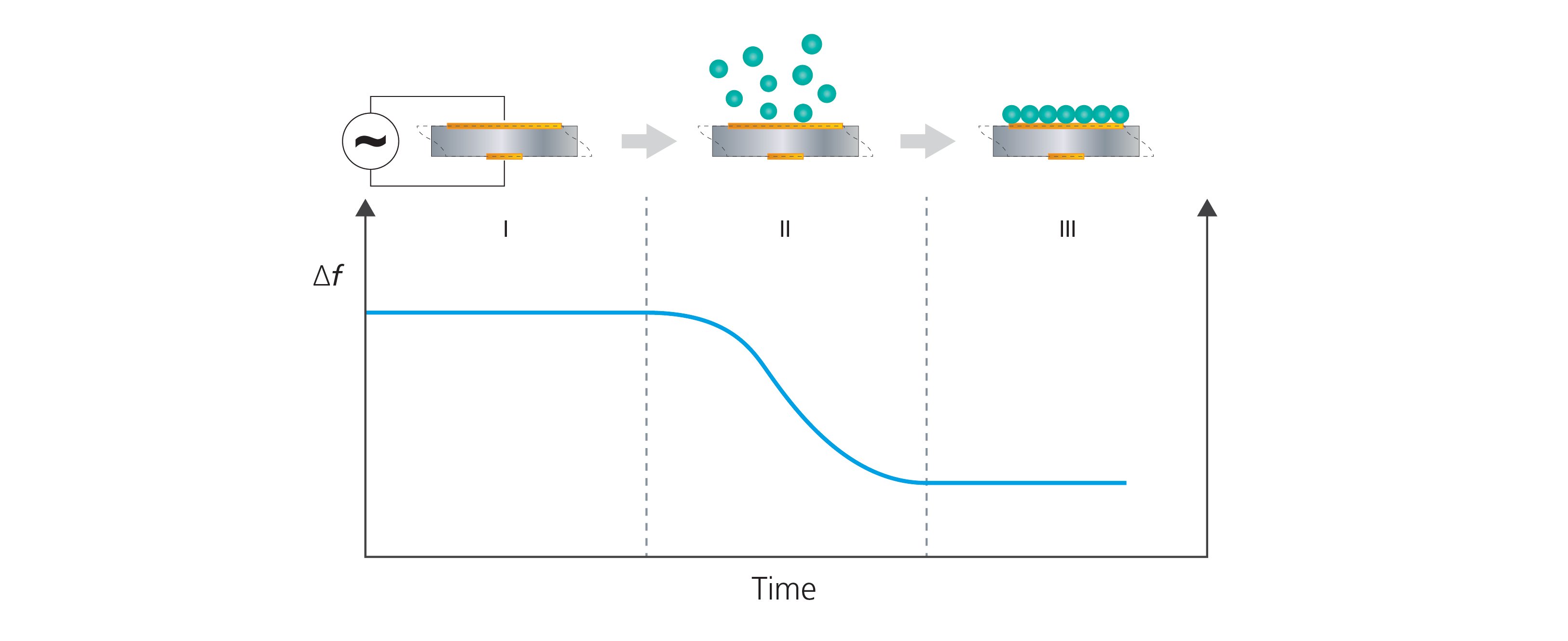 Figure 2. QCM technology collects time-resolved information on frequency changes of the QCM sensor and can detect mass uptake at the sensor surface. The schematic illustration shows (I) a bare sensor surface and a stable baseline of Δf. In step (II), molecules adsorb to the sensor surface which results in a frequency decrease. In (III), the surface uptake has been completed and the frequency response has stabilized. The frequency shift between I and III can be used to quantify the mass uptake at the sensor surface.
Figure 2. QCM technology collects time-resolved information on frequency changes of the QCM sensor and can detect mass uptake at the sensor surface. The schematic illustration shows (I) a bare sensor surface and a stable baseline of Δf. In step (II), molecules adsorb to the sensor surface which results in a frequency decrease. In (III), the surface uptake has been completed and the frequency response has stabilized. The frequency shift between I and III can be used to quantify the mass uptake at the sensor surface.
By following the changes in Δf, molecule – surface interaction can be detected and mass uptake and release from the surface can be quantified, Fig 3.
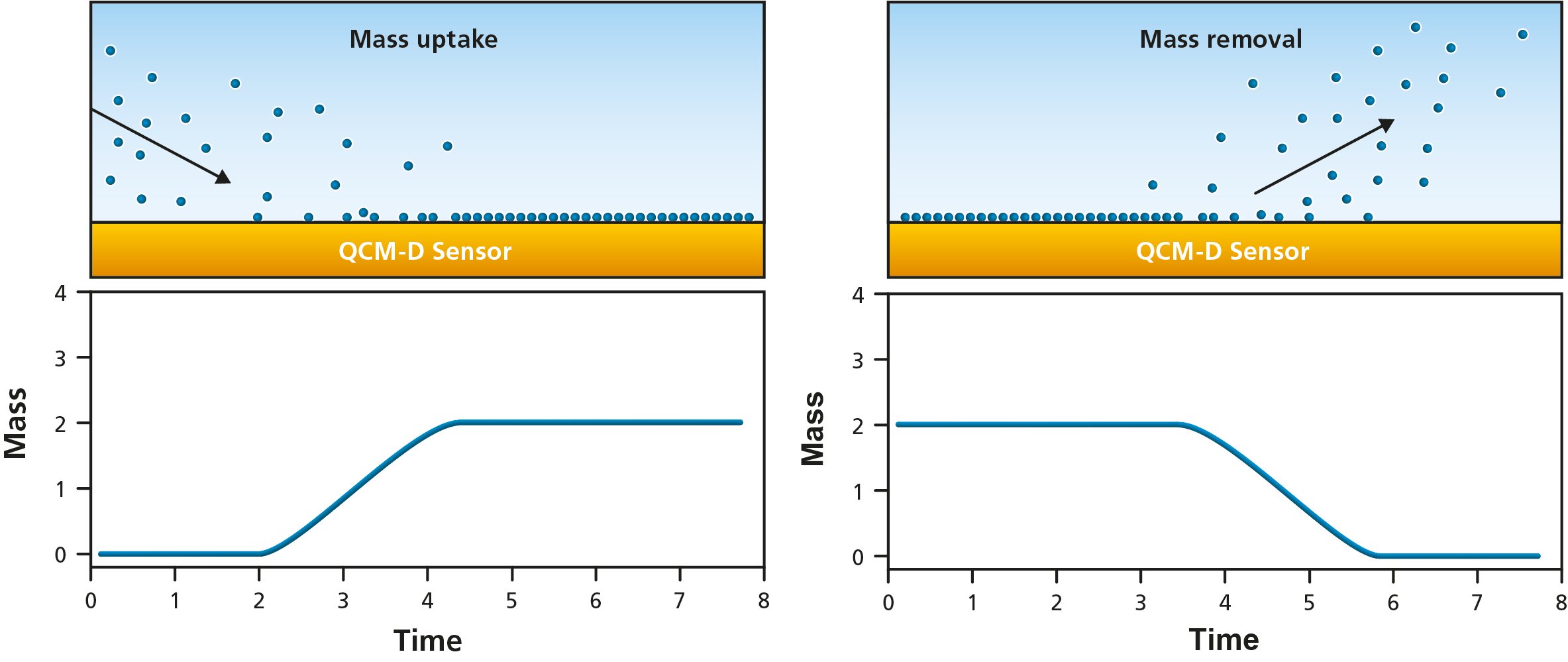 Figure 3. Schematic illustration of molecules (left) adsorbing to and (right) desorbing from the QCM sensor surface. The bottom panels illustrate the resulting changes in quantified mass, i.e., mass uptake as molecules adsorb and mass loss as they leave the surface.
Figure 3. Schematic illustration of molecules (left) adsorbing to and (right) desorbing from the QCM sensor surface. The bottom panels illustrate the resulting changes in quantified mass, i.e., mass uptake as molecules adsorb and mass loss as they leave the surface.
In summary, QCM technology is a real-time, surface-sensitive technique that monitors the change in mass or thickness of layers adhering to the surface of the QCM sensor. This is achieved by measuring the change in resonance frequency of the quartz crystal upon excitation by a driving voltage.
Download the overview to learn more about QCM technology and how it works.
Editor’s note: This post was originally published in January 2018 and has been updated for comprehensiveness
Learn how QSense QCM-D reveals protein–surface interactions and adds interface-focused insight to biopharmaceutical formulation and stability work
Learn how QSense sensors enable application‑relevant biointerface interaction analysis and explore our sensor offering for different areas
Learn how QSense QCM D can be used to analyze swelling of thin films, including magnitude and dynamics.
Read about how molecule-surface interaction processes such as adsorption and desorption can be analyzed with QCM-D.
Learn best practices and step-by-step methods for accurate QCM-D coating thickness measurement on QSense sensors using QSense Omni.
Compared to QCM, QCM-D measures an additional parameter, and provides more information about the system under study.
Discover how QCM-D analysis reveals real-time etching dynamics, helping optimize cleaning processes and protect surfaces from unwanted damage.
Discover how QSense QCM-D helps tackle fouling challenges across industries
Discover how QCM-D enables real-time, label-free analysis of supported lipid membrane formation, structure, and dynamics for advanced research
Learn how QSense QCM-D analysis can reveal membrane fouling dynamics and optimize cleaning strategies for more efficient water treatment
Learn how QSense QCM-D helps detect and prevent surface-induced instabilities in biologics. Join our webinar for insights and practical examples.
