
QSense QCM‑D measures adsorption and desorption in real time by recording shifts in resonance frequency (Δf) and dissipation (ΔD) of a quartz sensor. The frequency shift reflects mass uptake or loss on the surface, and the dissipation shift reflects the mechanical (viscoelastic) properties of the adsorbed layer.
Adsorption and desorption are important interfacial phenomena that often impact the performance and success of products and processes in many areas such as biotechnology, pharma, chemicals and cleaning products, as well as water and environmental technologies. In each of these areas, which molecules end up at the surface, when, in what amount, and for how long can have a major impact on whether a biomaterial becomes biocompatible or fouls, whether a biopharmaceutical remains stable, whether a cleaning product removes residues efficiently, or whether a water filter maintains its performance over time.
Because adsorption and desorption are both frequent and important at many interfaces, it can be valuable to study them to increase understanding of the system, product or process of interest. Surface and interface analysis techniques such as QSense QCM‑D, an acoustic, surface‑sensitive and label‑free technology, make these interfacial phenomena visible in real time and provide time‑resolved, quantitative data on mass uptake, mass loss and layer properties, supporting both fundamental insight and applied optimization. In simple terms, QCM-D acts as a very sensitive balance for small masses at the surface, recording how much material is added or removed and how the properties of the interfacial layer change over time.
When you run a QCM‑D experiment, the instrument records two primary signals:
Together, Δf and ΔD provide time‑resolved information about how much material is added to or removed from the surface, how fast this happens, and how the nature of the surface‑bound layer changes over time.
Depending on the application and objective of your study, it may be relevant to:
In all these cases it is useful to:
As material is added to or removed from a surface, there is a corresponding change in mass, which will be detected by QCM‑D in real time.
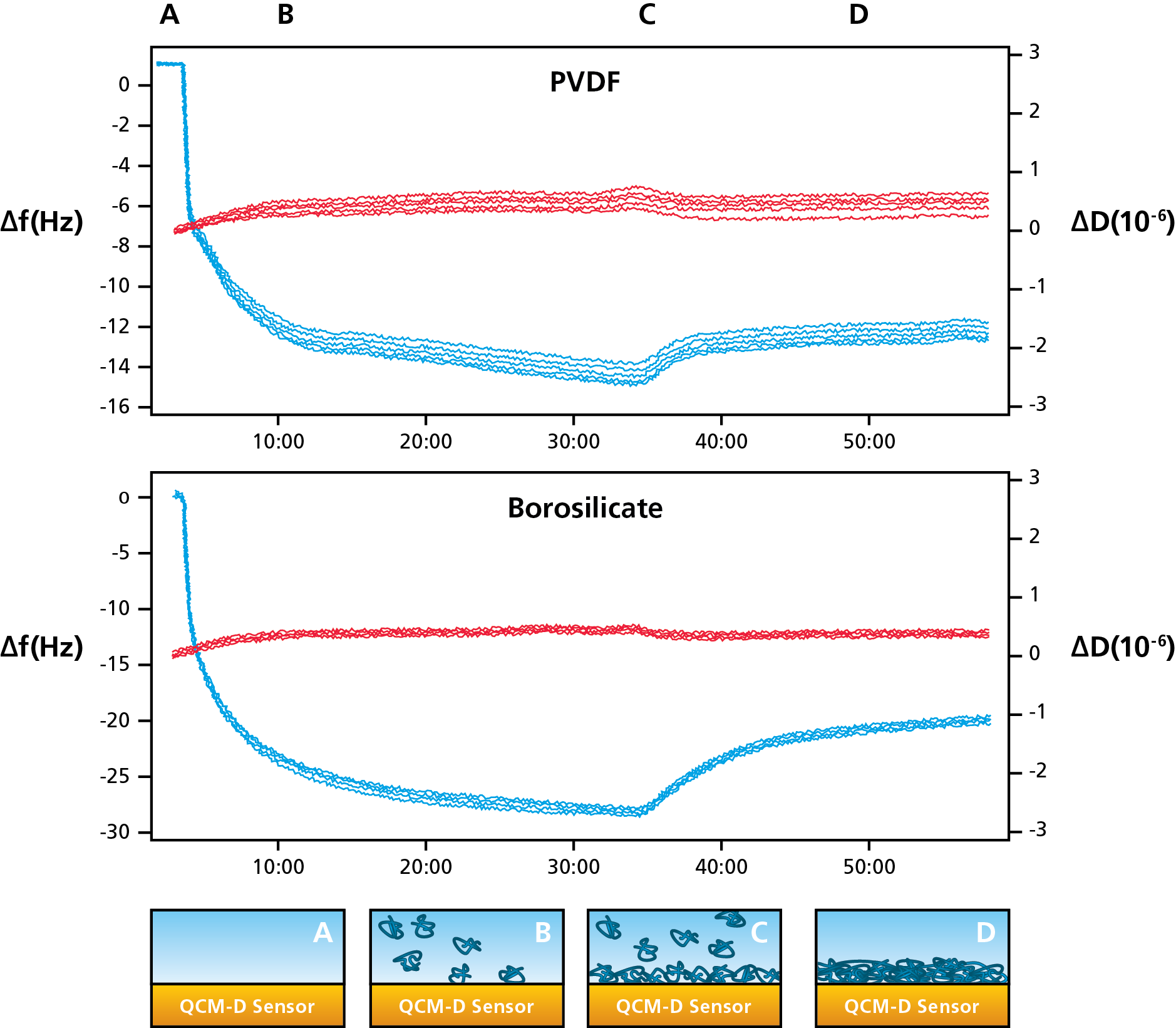
Figure 1. (Top) Protein adsorption on plastic (PVDF) and glass (borosilicate) measured with QCM-D. (Bottom) Schematic illustration of the protein adsorption process.
Below is a general protocol that can be adapted to your specific system.
When interpreting adsorption and desorption data, a few simple guidelines are useful:
Because QCM‑D is label‑free and time‑resolved, it is straightforward to compare adsorption and desorption behavior under different conditions, for example by varying:
This enables systematic studies of how environment and formulation changes affect surface interactions and layer stability.
In addition to the protein example shown here, many other adsorption and desorption events can be characterized by measuring mass uptake and mass loss with QCM‑D, including for example:
In all these cases the core readout is the same: time‑resolved changes in mass and viscoelastic properties of a layer on a sensor surface.
QSense QCM‑D provides a straightforward and sensitive way to monitor adsorption and desorption processes on solid surfaces. By following the mass (Δf) and dissipation (ΔD) in real time, you can quantify how much material adsorbs or desorbs, how fast it happens, whether it is reversible, and how the structure of the formed layer changes. This information is valuable across a wide range of research and development contexts where surfaces and interfaces play a critical role in product and process performance.
Download the overview to read more about what information you can obtain with QSense QCM-D.
FAQ
Q: How does QCM‑D measure adsorption?
A: QCM‑D measures adsorption as a decrease in resonance frequency (Δf↓) when mass accumulates on the sensor surface, and it records dissipation (ΔD) to indicate whether the adsorbed layer is soft or rigid.
Q: How does QCM‑D measure desorption?
A: Desorption is detected as an increase in frequency (Δf↑) when mass is removed from the surface during rinsing or exposure to a desorbing solution, often together with a decrease in dissipation as a softer layer is partly removed.
Q: What information can I get besides mass change?
A: In addition to mass uptake and loss, QCM‑D provides information about the viscoelastic properties of the interfacial layer via dissipation (ΔD), and about dynamics from the time‑resolved Δf and ΔD curves.
Q: Which other systems can I study with QCM‑D?
A: You can use QCM‑D to study adsorption and desorption of molecular and particulate systems forming interfacial layers in the nm to µm thickness range, such as lipids, proteins, polymers, surfactants, nanoparticles and more.
Q: What other processes can I study with QCM‑D?
A: In addition to adsorption and desorption, you can use QCM‑D to study interfacial phenomena such as binding, fouling, cleaning, film formation and film removal in many different application areas.
Editor’s note: This post was originally published in February 2018 and has been rewritten for comprehensiveness
Learn how QSense QCM-D reveals protein–surface interactions and adds interface-focused insight to biopharmaceutical formulation and stability work
Learn how QSense sensors enable application‑relevant biointerface interaction analysis and explore our sensor offering for different areas
Learn how QSense QCM D can be used to analyze swelling of thin films, including magnitude and dynamics.
Learn best practices and step-by-step methods for accurate QCM-D coating thickness measurement on QSense sensors using QSense Omni.
Compared to QCM, QCM-D measures an additional parameter, and provides more information about the system under study.
Discover how QCM-D analysis reveals real-time etching dynamics, helping optimize cleaning processes and protect surfaces from unwanted damage.
Discover how QSense QCM-D helps tackle fouling challenges across industries
Discover how QCM-D enables real-time, label-free analysis of supported lipid membrane formation, structure, and dynamics for advanced research
Learn how QSense QCM-D analysis can reveal membrane fouling dynamics and optimize cleaning strategies for more efficient water treatment
Learn how QSense QCM-D helps detect and prevent surface-induced instabilities in biologics. Join our webinar for insights and practical examples.
