Contact angle measurement is the most useful method to characterize the wettability of a solid surface. It provides the most surface-sensitive, quantitative, low-cost, and easy-to-conduct measurements. For these reasons, contact angle measurements are widely utilized in many industrial areas. The apparent simplicity of the measurement is however sometimes misleading, and care should be taken when contact angle measurements are conducted to be able to draw the correct conclusion.
One clear problem is that there is not just one unique contact angle for each surface. Instead, one can measure static contact angles, advancing and receding contact angles, and roughness corrected contact angles. Each of these can show different values on the same surface and sometimes it might be confusing which one to measure. Then on the other, while one of the contact angles might not correlate with the problem at hand, the other could be more suitable to solve it.
In this blog post series, different contact angles are discussed to understand how to utilize them in different applications.
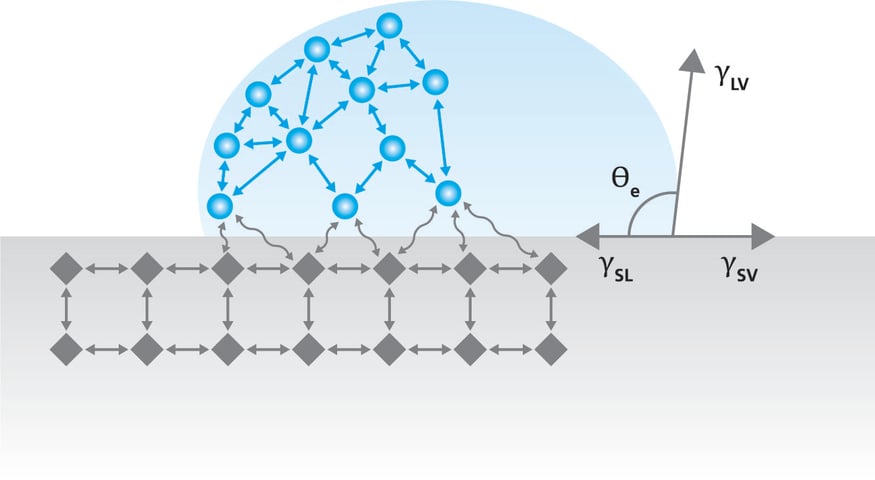
The static contact angle is a measure of the angle at which a liquid droplet meets a solid surface. 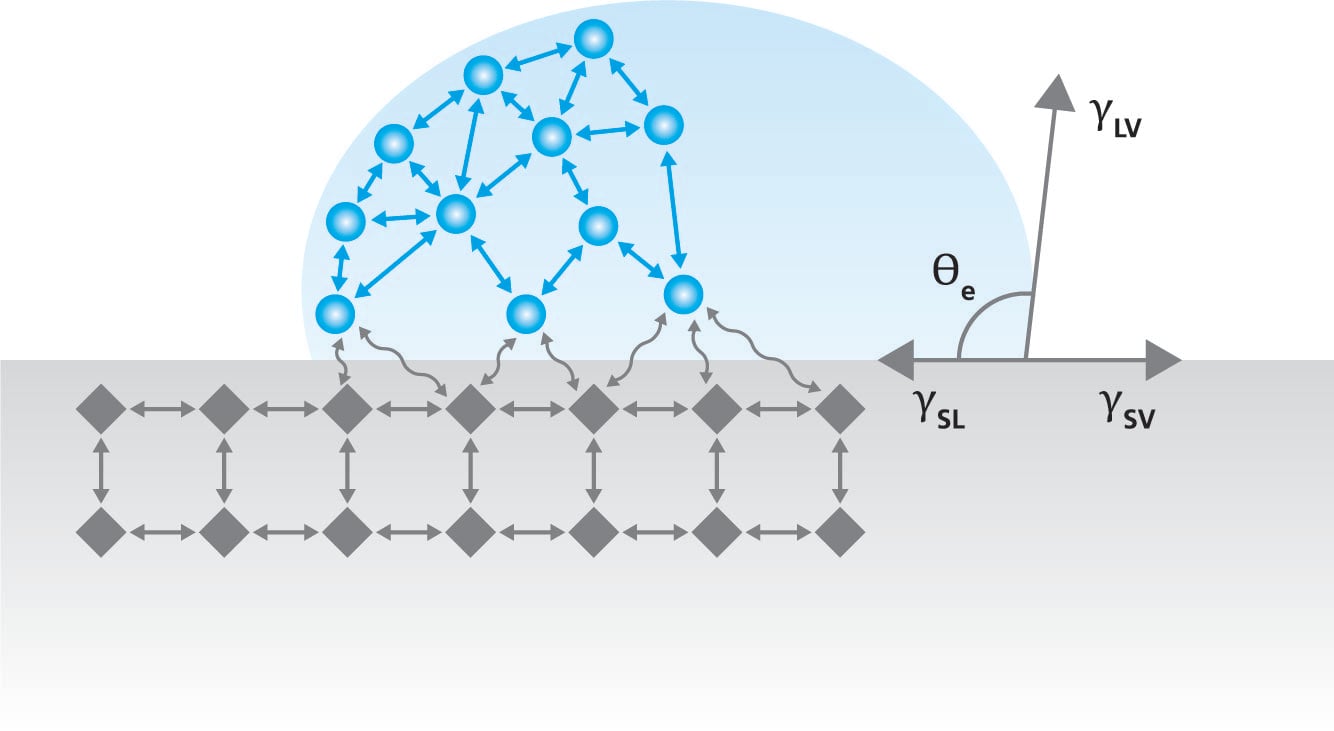 It is a measure of the wettability of the surface and is easily determined with the optical tensiometer. When static contact angle is measured, a drop of liquid is placed on a solid surface typically by using a manual or automated syringe. The droplet will take a shape on a solid which is determined by the interactions between the solid, liquid, and the surrounding phase, most often air. This interaction was expressed by Young over 200 years ago and it’s a foundation of contact angle and surface free energy theories still used.
It is a measure of the wettability of the surface and is easily determined with the optical tensiometer. When static contact angle is measured, a drop of liquid is placed on a solid surface typically by using a manual or automated syringe. The droplet will take a shape on a solid which is determined by the interactions between the solid, liquid, and the surrounding phase, most often air. This interaction was expressed by Young over 200 years ago and it’s a foundation of contact angle and surface free energy theories still used.
The static contact angle is often used to determine the hydrophilicity/phobicity of the surface. If the contact angle measurement is done with water, the surface is termed hydrophilic when the contact angle is lower than 90 degrees and hydrophobic when it’s above.
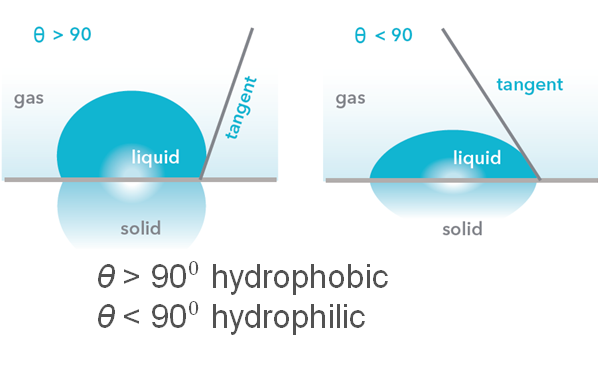 There are several applications for both hydrophilic and hydrophobic surfaces and a static contact angle can be useful when a material is selected for such an application.
There are several applications for both hydrophilic and hydrophobic surfaces and a static contact angle can be useful when a material is selected for such an application.
The static contact angle can be useful in quality control e.g. evaluating the effect of surface treatment on contact angle. For example, if it is known that successful plasma treatment lowers the contact angle from over 90 degrees below 30, contact angle provides an easy method to check that.
One of the most common usages of static contact angle is for the calculation of surface free energy. Surface free energy is a property of the solid surface. Surface free energy is measured as one of the prerequisites for wettability is that the surface free energy of the solid is higher than the surface tension of the coating formulation. Thus, surface free energy is often calculated and the value is compared with the surface tension of different coating formulations to determine the best formulation candidate.
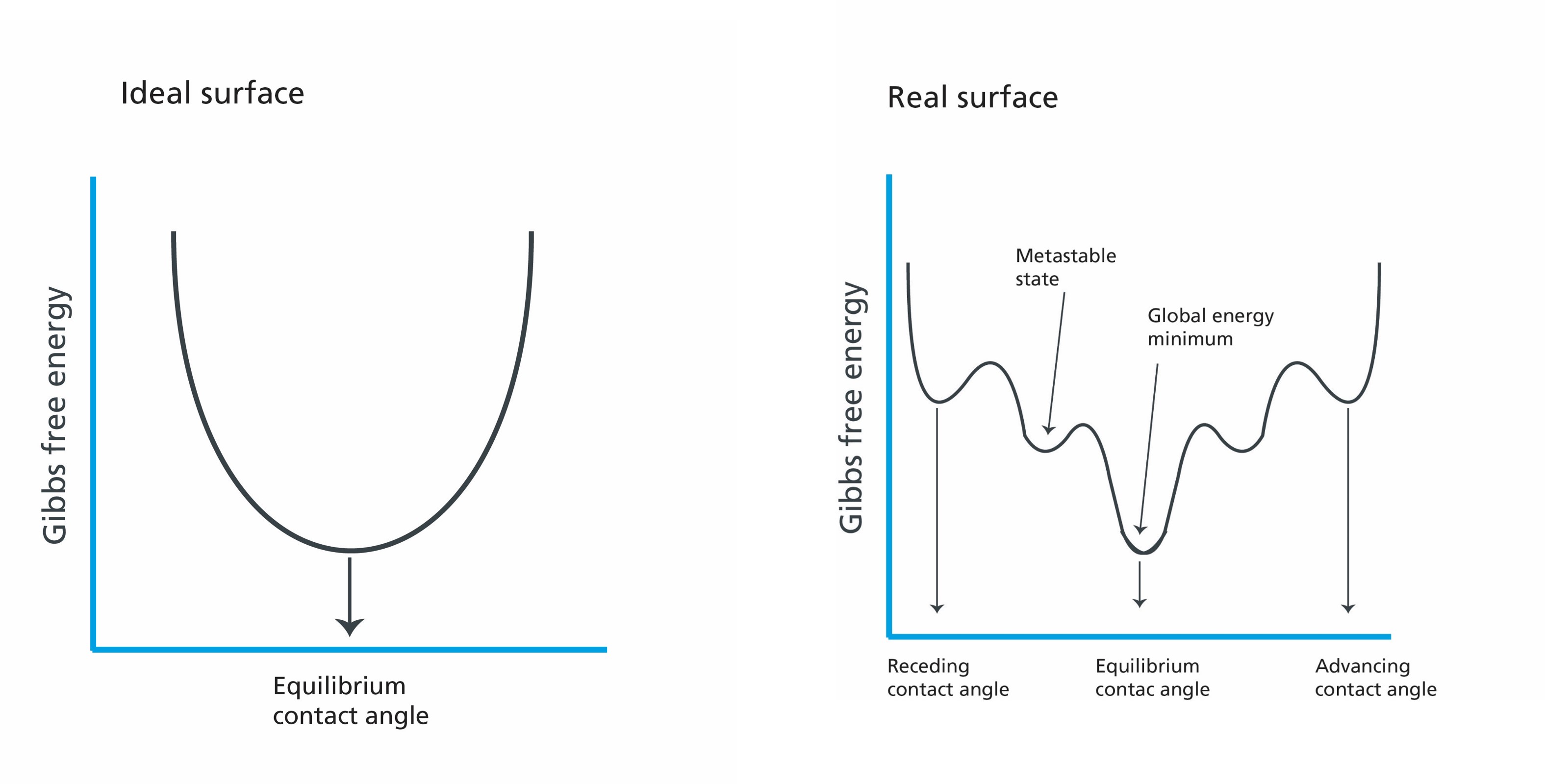
Although the measurement of static contact angle is seemingly simple, there are several problems associated with it. The main problem can be most easily explained with the Gibbs free energy sketch for the ideal and real surface. If the surface would be ideal, there would be only one energy minimum at which the droplet would be. The contact angle measured would then be equivalent to this energy minimum and could be termed equilibrium contact angle. On real surfaces, however, there are several local minimums with energy barriers in between them. This means that on real surfaces, the droplet can take any shape in between these, and it is not necessarily equivalent to a global energy minimum. One can thus measure several contact angle values on the same surface, the topic which is further discussed in the upcoming posts of the blog series.
If you would like to read more about contact angle and its measurement techniques, please download the white paper through the link below.
Learn how to use contact angle measurements to evaluate surface cleanliness on different materials and link cleaning steps to reliable adhesion and coating quality.
Learn how wettability, surface free energy and surface roughness define paper and paperboard surface properties – and how to measure them for reliable print, coating and sealing performance.
Smart materials are materials that sense a change in their environment and respond in a useful, reversible way.
Discover how contact angle measurements help optimize plasma treatment for improved wettability and adhesion in industrial applications.
Discover why contact angle is essential for adhesion, coatings, and quality control. Learn how surface wettability impacts product performance.
Discover why PFAS-free coatings are needed, the challenges they present, and key strategies for developing high-performance alternatives.
At the heart of droplet formation are two key molecular forces: cohesion and adhesion.
Contact angle measurements provide a golden standard for evaluation of surface properties for quality control.
Contact angle is the angle a droplet forms in contact with a solid surface. Thermodynamically, it is a balance between cohesive and adhesive forces.
