A Langmuir-Blodgett film (or LB film) can be defined as one or more monolayers of material deposited from a liquid surface onto a solid substrate by dipping the substrate through a floating monolayer at a constant molecular density. LB films are formed by one or several Langmuir films deposited onto a solid surface by vertical dipping of the solid substrate from the gas phase into the liquid phase (or vice versa). The name Langmuir-Blodgett comes from its two inventors Irvine Langmuir and Katharine Burr Blodgett.
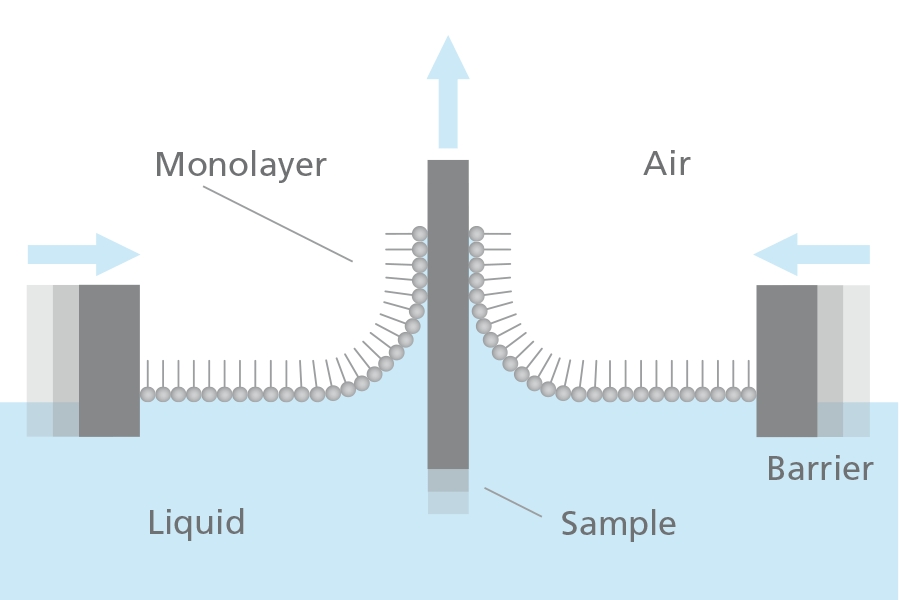
Langmuir-Blodgett technology enables the deposition of single- or multimolecular layers from a liquid surface onto a solid substrate with excellent film structure control. 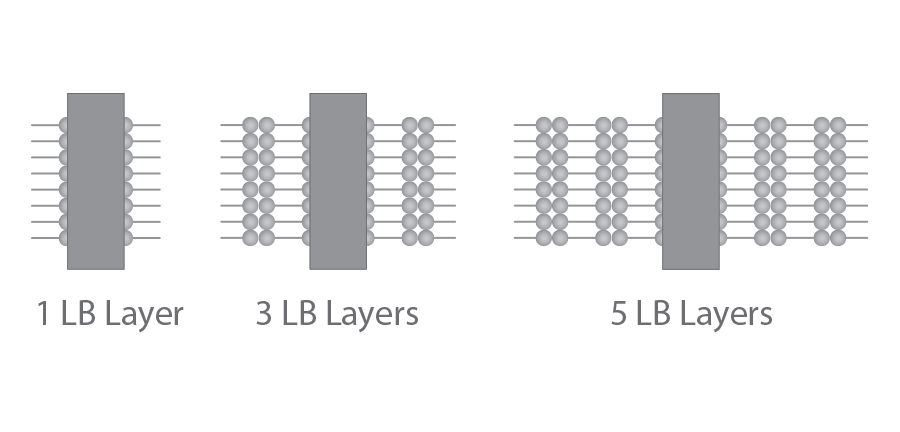 LB is suited especially well for example for creating highly organized nanoparticle coatings.
LB is suited especially well for example for creating highly organized nanoparticle coatings.
The films obtained by this process can be highly organized, ranging from ultrathin monolayers to multilayer structures built up of hundreds of monolayers.
Most typical applications of LB include creating highly organized and controlled nanoparticle coatings on solid substrates. These coatings can be used as an end product for instance in electronics, biomaterials, sensors, or functional surfaces.
Repeated deposition can be used to create well-organized multilayers on solid substrates. Several parameters affect the type of LB film produced. These include the nature of the spread film, the sub-phase composition, and temperature, the surface pressure during the deposition and the deposition speed, the type and nature of the solid substrate, and the time the solid substrate is stored in air or the sub-phase between the deposition cycles.
Density, thickness, and homogeneity properties are preserved when transferring the Langmuir film onto the substrate, allowing the construction of organized multilayer structures with varying layer compositions.
If you would like to learn more about Langmuir and Langmuir-Blodgett films and their applications, please register for the user event through the link below.
Surface pressure—area isotherm can be defined as a measurement at a constant temperature of surface pressure, as a function of the available area for each molecule in Langmuir film.
Learn more about what information QCM-D, Quartz Crystal Microbalance with Dissipation monitoring technology, offers
The versatility of polyelectrolyte multilayers, PEMs, is high, which makes them interesting for e.g. biomedical applications. The functionality is largely determined by the layer properties, which needs to be understood to be tailored. Here, we show how PEMs can be characterized with QCM-D.
Polymers and polyelectrolytes of various conformations are used in many applications where there is a need to tailor the interfacial properties to promote a certain interaction with the surrounding environment. Here we show how polymer layer crosslinking and collapse can be characterized.
Read about how molecule-surface interaction processes and binding can be characterized by QCM-D via time-resolved measurements of mass and thickness.
Thin-film degradation and material desorption is sometimes wanted and sometimes not. Learn about how it can be characterized by QCM-D.
Surfaces are all around us. To control the macroscale we need to understand and tune the nanoscale. Learn more about QCM-D can help.
